The role of digital technologies in tackling the Zika outbreak: a scoping review
Introduction
The 2015/2016 Zika outbreak is considered as one of the major global health crises of the 21st century. The origins of the outbreak can be traced back to French Polynesia in 2013 (1). Zika virus (ZIKV) spread rapidly in the Pacific islands and reached Brazil in 2015 (1,2).
The primary transmission route of ZIKV is vector-borne through the bite of an infected mosquito (Aedes genus, mainly Aedes aegypti) and has an incubation period of 3–14 days post-exposure (3,4). Furthermore, infected pregnant women can transmit ZIKV directly to the foetus; whilst it can also be transmitted through sexual contact with an infected individual (3,5). Infection is predominantly asymptomatic (5) or with only mild symptoms, which include fever, skin rash, conjunctivitis, muscle and joint pain, malaise and headache (3,5). ZIKV disease is usually mild and does not require specific treatment, with symptoms lasting only 2–7 days (3). However, after increased reporting of growing clusters of children born with microcephaly and other neurological disorders in Brazil, in February 2016 World Health Organisation (WHO) declared it a Public Health Emergency of International Concern (PHEIC) (6). Similar cases of microcephaly were also retrospectively reported in French Polynesia in 2014, and continued to spread through the Americas and Pacific throughout 2015–2016 (6). ZIKV RNA was detected in the amniotic fluid of affected newborns. After direct transmission from an infected pregnant woman to her foetus was confirmed (6), ZIKV was established to cause severe neurological complications in foetuses and neonates (6). Associations between ZIKV and Guillain-Barré syndrome (GBS) in adults and microcephaly in newborns were reported in Brazil in 2015 (7). As a result, WHO concluded that ZIKV infection during pregnancy can cause congenital brain anomalies, such as microcephaly; and further could be a causal agent for GBS (3).
In addition to pregnant women, travellers are a population group of high concern in the transmission of ZIKV. Although WHO was quick to identify the Zika outbreak a PHEIC, in less than a year since the declaration, the virus had spread to more than 25 nations in the Americas, the Pacific islands, and West Africa (8). As of May 2016, Brazilian national authorities had estimated 500,000 to 1500,000 cases of Zika; Colombia had reported 56,477 suspected cases; and Cabo Verde (Africa) had reported 7,499 as of March 2016 (7). Globally, approximately 1.6 million autochthonous cases of Zika have been reported as of March 2016 (9). Similarly, Brazilian authorities reported a total of 13,914 suspected cases of microcephaly and other congenital central nervous system (CNS) malformations between epidemiological week (EW) 32 of 2015 and EW 22 of 2017, of which there were 2,775 confirmed cases of microcephaly associated with ZIKV infection (10).
As Zika developed into a PHEIC, exploring new ways of predicting and monitoring its spread became crucial given the limitations of traditional infectious disease monitoring methods. A criticism of the Brazilian response to the outbreak was that they were unable to effectively fight and win the battle against mosquitos due to the implementation of inadequate traditional control strategies, such as the deployment of the army to conduct anti-mosquito campaigns in houses (11). In contrast, innovative approaches such as the deployment of Wolbachia-infected mosquitoes, which can limit transmission of ZIKV promise much greater efficacy (12) but the impact of such interventions require thorough assessment. In this instance, the use of computational predictive models could simulate the impact of such interventions on mosquito populations, indeed, such models are already used to inform Dengue fever control strategies (13). As a result of the clear potential benefits they represent, the use of digital technologies for disease outbreak interventions has increased (14). Public health challenges of this nature require the development and use of innovative approaches, such as digital technologies, which can assess, predict, monitor and control the spread and transmission of the disease. The term ‘digital technologies’ may refer to a variety of digital resources which are used to gather disease-related data, including but not confined to; mHealth (e.g., cell phones, mobile devices); global positioning system (GPS) [e.g., geographic information system (GIS)]; social media (e.g., Facebook, Twitter); remote sensing technologies (e.g., wireless remote health monitoring system, satellite imaging); and electronic management databases (e.g., computer-based modelling). An example of digital technology is the use of an affordable and portable molecular device for rapid point of care (POC) diagnosis of ZIKV (15). The device could replace the conventional immuno-based rapid test, which is often not successful in distinguishing between Zika and other related Flaviviruses (15).
This review focuses on the recent Zika outbreak because it presented a key opportunity for digital technologies to demonstrate their value during a public health crisis. Digital technologies have the potential to improve disease monitoring, prevent and control cross-border transmission, and improve the timeliness of outbreak discovery, and awareness the public engagement. One such example of this approach is Digital Participatory Surveillance, a web-based disease monitoring system to support and improve global disease outbreaks management through encouraging members of the public to report signs and symptoms of their infections (14). However, little is known about the role of digital technologies in managing global emerging infectious diseases outbreaks as a whole, hence the rationale for this work.
Study purpose and research question
The purpose of this scoping review was to summarize the current state of research activity, by extent and by nature, regarding the utilization and the role of digital technologies during the Zika outbreak by providing an overview of publications covering this subject. Therefore, this scoping study attempted to answer the following research question:
- To what extent is the subject covered in the scientific literature between January 2016 and July 2017?
- What types of articles (e.g., reviews, original research) are published on the topic?
- What is the geographical focus of the publications?
- What is known about the utilization of digital technologies with specific reference to the 2016 Zika outbreak?
Methodology
Based on the scoping review methodology (16) as defined by Arksey and O’Malley (16), that scoping reviews are used “to map rapidly the key concepts underpinning a research area and the main sources and types of evidence available”. A key strength of this method, as applied to our review, is that it allows for the analysis of broad research questions. The utilisation of digital technologies to tackle the Zika outbreak is a vast area of research and our intention was to map the breadth of the research activity on this topic. Hence, we reviewed the published literature on the use of digital technologies in the context of the 2016 Zika outbreak. Using the following methods, we collated published literature, extracted data, and undertook a descriptive analytical method to summarize the information. We mapped the findings by categorizing the publications based on their relevance to a specific digital technology domain (Table 1).
Table 1
| Digital technology domain | Description |
|---|---|
| Computational modelling | Models involve assumption, abstraction and simplification, of complex disease-associated dynamics (17). This review covered modelling as computer/software assisted modelling, primarily referring to mathematical, computational, spatial-temporal modelling |
| Big data | A term describing the storage and analysis of large and or complex data sets using a series of techniques including, but not limited to: cloud computing, non-relational databases, natural language processing and machine learning (18). This review covered big data mostly through big data analytics of social media data (e.g., Twitter, Facebook, YouTube, Instagram), and web-based surveillance (HealthMap, ProMed, EpiCore, and Digital Participatory Surveillance) |
| mHealth | mHealth refers to medical and public health practices supported by mobile devices, such as mobile phone technology, patient monitoring devices, personal digital assistants, and other wireless devices (19). This review covered mHealth through the use of mobile phone applications |
| Novel technologies | Case-specific technologies produced or updated, to specifically track and monitor the outbreak, considered “interestingly new or unusual”. This review included nanotechnologies using nano-magnetic materials and methods, amongst other case-specific technologies |
Identifying relevant studies and search strategy
In order to comprehensively identify relevant studies in the field, we developed and implemented a comprehensive search strategy. The search strategy combined English related terms of digital technology and the Zika outbreak containing a combination of free text and PubMed MeSH terms. Our search strategy, in summary, included, but not confined to; (Zika OR “Zika virus infection” OR “ZIKV” OR “Zika virus disease” OR “viral disease”) AND (“Digital” OR “Technology” OR “Precision medicine” OR “Biosensor” OR “Sensors” OR “Bio-surveillance” OR “Intelligent surveillance”). These search terms were used to identify relevant literature in two main databases, PubMed and Web of Science (see complete search syntax in Table S1).
Study selection
The scoping review included all article types namely, original quantitative and qualitative approaches, systematic reviews, editorials and viewpoints, indexed in PubMed or Web of Science databases. The publication dates were restricted to period January 2016 to July 2017. This date range was selected to cover the period when Zika outbreak as a PHEIC started and ended in Brazil (May 2017).
The collected articles were independently screened by two reviewers (S Ahmadi & NE Bempong) for relevance to the inclusion and exclusion criteria. We first screened the published papers by reviewing their titles and abstracts. The papers not meeting the inclusion criteria were excluded. A final selection was made by reading the full-texts. The two reviewers (S Ahmadi & NE Bempong) met several times at the beginning, midpoint, and final stages of the abstract selection process to discuss agreements or disagreements, challenges faced, and uncertainties related to study selection. We went back and refined the search strategy as needed. In case of disagreement, a third reviewer (A Flahault) was consulted to determine final inclusion.
Charting, collating and summarizing data
In the analysis stage, a descriptive numerical summary was provided which included the following information from the focal articles: author/s, publication year, study type, geographic region of the study, digital technology domain, functions served by each technology, and main results (Table 2). The geographic region of the papers was determined based on the WHO regional grouping, namely; African region, region of the Americas, South-East Asia region, European region, Eastern Mediterranean region, and Western Pacific region. Papers that did not focus on a specific region or country, or focused on more than one region were classified into “others”. The extracted data was extrapolated into a data charting form in an excel file. The two reviewers independently extracted data for the first five studies using the data-charting form and met to determine whether their approach to data extraction was consistent with the research question and purpose.
Table 2
| Digital technology domain | Geographical region | Study approach | Study topic | Description | Function | Reference |
|---|---|---|---|---|---|---|
| Computational modelling | Others | Spatial/GIS analysis | ZIKV outbreak epidemiological maps | The potential magnitude of the ongoing Zika epidemic: 1.65 (1.45–2.06) million childbearing women and 93.4 (81.6–117.1) million people in total could be infected in the Americas | Monitoring | (20) |
| Americas | Spatial/GIS analysis | ZIKV outbreak epidemiological maps | ZIKV incidence rates | Monitoring | (21) | |
| Americas | Spatial/GIS analysis | ZIKV outbreak epidemiological maps | ZIKV incidence rates | Monitoring | (22) | |
| Americas | Spatial/GIS analysis | ZIKV outbreak epidemiological maps | ZIKV incidence rates | Monitoring | (23) | |
| Americas | Spatial/GIS analysis | ZIKV outbreak epidemiological maps | ZIKV incidence rates | Monitoring | (24) | |
| Americas | Spatial/GIS analysis | ZIKV outbreak epidemiological maps | ZIKV incidence rates | Monitoring | (25) | |
| Others | Spatial/GIS analysis | ZIKV transmission dynamics | Identified areas that are environmentally suitable for ZIKV transmission | Monitoring | (26) | |
| Americas | Mathematical modelling | ZIKV transmission dynamics | Real-time estimation of transmission dynamics during the 2015–2016: estimated basic reproduction number (Ro) using different data sources | Monitoring | (27) | |
| Western Pacific region | Mathematical modelling | ZIKV transmission dynamics | Basic reproduction number ranged from 2.6–4.8, with an estimated 11.5% (95% CI: 7.32–17.9%) of total infections reported | Monitoring | (28) | |
| Americas | Mathematical modelling | ZIKV transmission dynamics | Basic reproduction number calculated (R0: 2.055) (95% CI: 0.523–6.300), in which the percentage contribution of sexual transmission is 3.044% (95% CI:0.123–45.73) | Monitoring | (29) | |
| Others | Mathematical modelling | ZIKV transmission dynamics | Countries at risk of mosquito-borne ZIKV infection include India, China, Indonesia, Philippines, and Thailand | Monitoring | (30) | |
| Americas | Mathematical modelling | ZIKV transmission dynamics | Temperature-dependent transmission is an important predictor of human transmission | Monitoring | (31) | |
| Americas | Mathematical modelling | ZIKV transmission dynamics | The country-level risk for Zika transmission: Important risk concentrated in metropolitan areas | Monitoring | (32) | |
| Others | Mathematical modelling | ZIKV transmission dynamics | The ZIKV outbreak in Latin America has very likely been fueled by the 2015–2016 El Nino climate phenomenon affecting the region | Monitoring | (33) | |
| Others | Mathematical modelling | ZIKV transmission dynamics | Disease transmission dynamics of ZIKV through sexual transmission route: the sexual transmission route contributes significantly to the disease burden in the community | Monitoring | (34) | |
| Americas | Mathematical modelling | ZIKV transmission dynamics | Transitional parameters affected disease progression | Monitoring | (35) | |
| Americas | Mathematical modelling | ZIKV transmission dynamics | Epidemic outcomes of the model correlated with the spatially dependent parameters and initial conditions of the model | Monitoring | (36) | |
| Others | Mathematical modelling | ZIKV transmission dynamics | Infection dynamics from transport through popular places | Monitoring | (37) | |
| Others | Mathematical (niche) modelling | ZIKV dispersion and spread | Predicted global potential distribution of ZIKV | Monitoring | (38) | |
| European region | Mathematical modelling | ZIKV dispersion and spread | 508 and 1,778 estimated imported infections into Europe (mainly France, Portugal, Italy) in 2016 | Monitoring | (39) | |
| Americas | Spatial/GIS analysis | ZIKV dispersion and spread | Reconstruction of ZIKV introduction in Brazil: Southward pattern of spread of Zika starting from the northeastern coast and a pattern of movement toward the western border. Average speed 42 km/day or 15,367 km/year | Monitoring | (40) | |
| Americas | Mathematical modelling | ZIKV dispersion and spread | The first introduction of ZIKV to Brazil likely occurred between August 2013 and April 2014 | Monitoring | (41) | |
| Others | Spatial/GIS analysis | Vector: transmission risk distribution | Risk maps for vector transmission. Aedes mosquitoes likely to live year-round across many tropical areas in the Americas, Africa, and Asia | Monitoring | (42) | |
| Others | Mathematical (niche) modelling | Vector: transmission risk distribution | The vector transmission risk distribution. 2.261 billion world population identified under high and very high risk | Monitoring | (43) | |
| Others | Spatial/GIS analysis | Vector: transmission risk distribution | Aedes aegypti has greater global dispersion than the Aedes Albopictus species | Monitoring | (44) | |
| Americas | Mathematical modelling | Vector: population dynamics | Models that describe the mosquito's biological and ecological characteristics | Monitoring | (45) | |
| Western Pacific region | Mathematical modelling | Vector: Aedes aegypti-suppressing Wolbachia mosquitoes | Natural Aedes Aegypti populations could be rapidly transformed with disease-suppressing Wolbachia | Monitoring | (46) | |
| Americas | Mathematical modelling | Vector: potential ZIKV vectors | 35 mosquito species could potentially transmit ZIKV | Monitoring | (47) | |
| Americas | Mathematical modelling | Effective intervention strategies to control the spread of ZIKV | The effectiveness of containment strategies of the outbreak: reducing the biting rate, increasing vector mortality rate, reducing larvae carrying capacity, and reducing vector population | Monitoring | (48) | |
| Others | Mathematical modelling | Effective intervention strategies to control the spread of ZIKV | Effective interventions strategies for the ZIKV spread. Basic reproductive number | Monitoring | (49) | |
| Americas | Spatial/GIS analysis | Causal analysis: ZIKV infection and yellow fever vaccination | High yellow fever vaccination coverage among pregnant women may pose their offspring to lower risk to develop microcephaly | Monitoring | (50) | |
| Americas | Spatial data mining | Potential host-virus interaction | Ten mammals identified including bats as non-human hosts of ZIKV | Monitoring | (51) | |
| Big data | Others | Case study | Web-based surveillance | The OpenZika project started the virtual screening of 6 million compounds to discover novel candidate compounds for developing new drugs for treating ZIKV | Treatment | (52) |
| Others | Viewpoint | Web-based surveillance | Digital participatory surveillance (DPS). A form of digital surveillance where the public reports signs and symptoms | Monitoring | (14) | |
| Others | Review | Web-based surveillance | ProMed collects and disseminates data on outbreaks. One of the first outlets to report early Zika spread in the Americas in 2015 | Monitoring | (53) | |
| Others | Cross-sectional | Social media/internet (Google and Bing) analytics. Misinformation | Studied the issue of misinformation and pseudo-scientific claims about Zika on social media and internet | Monitoring | (54) | |
| Others | Content analysis | Social media (Twitter) analytics. Misinformation | Pseudo-scientific claims were being advanced by existing vaccine sceptic communities. 86% of these users tweeted about vaccines in 2015, and at least 19% of users tweeted an anti-Zika vaccine message | Monitoring | (55) | |
| Americas | Cross-sectional | Social media analytics. Social media (Facebook). Misinformation | Misleading posts were far more popular than the posts dispersing accurate, relevant public health information | Monitoring | (56) | |
| Americas | Experimental | Social media (Facebook) analytics. Misinformation | Algorithmic and social corrections are equally effective in limiting misperceptions, and correction occurs for both high and low conspiracy belief individuals | Monitoring | (57) | |
| Americas | Time series | Data streams/social media (Twitter) analytics | Strong positive correlations found between news (daily volume) and tweets. Also found strong positive correlations between news and Google searches | Monitoring | (58) | |
| Others | Content analysis | Social media (Twitter) analytics | Social media users were more concerned about issues such as pregnancy, abortion, microcephaly etc. than the symptoms such as fever, skin rash or red eye related to Zika disease | Monitoring | (59) | |
| Others | Content analysis | Social media (Twitter) analytics. Social media engagement of the public and CDC | Both the public and the CDC expressed concern about the spread of ZIKV, but the public showed more concern about the consequences it had for women and babies, whereas the CDC focused more on symptoms and education | Monitoring | (60) | |
| Others | Content analysis | Social media (Twitter) analytics. Social media engagement of the public | Zika-related Twitter incidence peaked after the WHO declared an emergency | Monitoring | (61) | |
| Western Pacific region | Cross-sectional | Social media (Facebook) analytics | Use of social media during Zika outbreak by national and international agencies for the purpose of health communication | Monitoring | (62) | |
| Others | Spatio-temporal analysis | Social media (Twitter) analytics | Use of social media during Zika outbreak by national and international agencies for the purpose of health communication. CDC and WHO had a prominent presence on Twitter discussions | Monitoring | (63) | |
| Others | Content analysis | Social media (Twitter) analytics | Social media engagement of the public. Description of topics twitted by the people about Zika on Twitter | Monitoring | (64) | |
| Others | Content analysis | Photo sharing social media analytics (Instagram and Pinterest) | 47% (290/616) of Pinterest photos and 23% of Instagram photos were relevant to ZIKV | Monitoring | (65) | |
| Americas | Content analysis | Video sharing social media analytics. Social media (YouTube) | 100 most viewed videos described on YouTube | Monitoring | (66) | |
| Americas | Cross-sectional | Social media analytics. Use for health monitoring | The overall low rate of social media monitoring reported | Monitoring | (67) | |
| Americas | Cross-sectional | Social media (Facebook and Twitter) and internet-based data stream analytics | Obstetric care practices do not optimally use their website to inform populations about the risk of ZIKV. 25% and 35% of obstetric practice websites had information posted about ZIKV in January 2016 and August 2016, respectively | Monitoring | (68) | |
| Americas | Predictive modelling | Different web-based data streams analytics | Tracked and predicted Zika incidence with data from Google trends, Twitter, HealthMap | Monitoring | (69) | |
| mHealth | Others | Case study | Smartphone app | Zika Tracker App: to report suspected or confirmed Zika cases | Monitoring | (70) |
| Others | Viewpoint | Smartphone app | WHO’s Zika App: provides information on diagnostic, surveillance, management of complications, and WHO response plan. | Monitoring | (71) | |
| Others | Case study | Smartphone app | A smartphone-based diagnostic platform for rapid detection of ZIKV | Diagnostic | (72) | |
| Others | Case study | Smartphone app | The reading of the results of a paper-based device for the detection of ZIKV was simplified by using a dedicated app | Diagnostic | (73) | |
| Novel technologies | Americas | Experimental | Wearable insecticide device | Studied the efficacy of some wearable insecticide devices compared with traditional repellent for Aedes aegypti | Treatment | (74) |
| Americas | Viewpoint | Portable genome-sequencing device | MinION, a real-time portable genome sequencing device was used in Brazil to perform portable whole-genome sequencing after tiling PCR | Monitoring | (75) |
app, application; CDC, Centers for Disease Control and Prevention; GIS, geographic information system; ZIKV, Zika virus.
Results
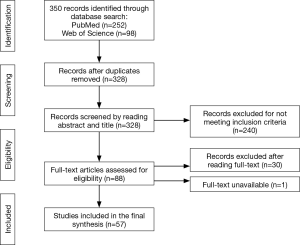
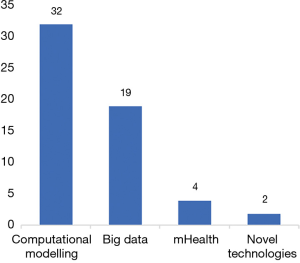
We screened 350 titles and abstracts, of which we identified 328 unique records after excluding duplicates (n=22). A total of 240 records did not meet the inclusion criteria (Figure 1). We then screened full-text records (n=88) for relevancy, leaving 57 published papers to be included in the final synthesis (Figure 1). Four main themes or digital technology domains were identified from these published papers (Figure 2). These domains are defined in Table 1. The 57 publications identified in this scoping review are classified and summarized in Table 2.
The majority of the articles (n=54/57) used quantitative methodology. The majority of the studies (n=55/57) used non-experimental study designs with only 2 articles reporting experimental approaches. In terms of geographical focus, approximately half (n=27/57) of the published papers targeted the region of the Americas (Table 2). Few papers targeted the Western Pacific (n=3/57) or European regions (n=1/57) while the rest of the papers (n=26/57) did not target a single particular geographic region.
Functions
Most of the studies (n=53/57) identified digital technologies that served the function of monitoring during the Zika outbreak. A small portion of studies addressed the topic of diagnosis (n=2/57) and treatment (n=2/57) of ZIKV.
Computational modelling
This scoping review identified 32 papers (n=32/57) that used computational modelling techniques including mathematical modelling (n=20/57) and geographical information systems (GIS) (n=12/57) mapping-based approaches mainly to estimate the magnitude of the Zika outbreak, study transmission dynamics, and predict the spread of ZIKV to other regions.
ZIKV incidence rates and the potential magnitude of the outbreak were estimated with the use of epidemiological maps (20-25). ZIKV transmission dynamics studies (26-29,31-37) estimated the basic reproduction number for the outbreak (27-29); identified geographic areas at risk for transmission (26,30,32,37) and predictors of transmission (31); estimated the burden of the sexual transmission route (34) and modelled the outbreak or infection dynamics (33,35-37). Some studies focused on the estimation of ZIKV global dispersion and spread (38,40,41) including importation to Europe (39).
Mathematical and spatial modelling techniques were also used to increase knowledge on the Aedes spp. vectors. A modelling study predicted 35 mosquito species that could potentially transmit ZIKV (47). The global vector transmission risk distribution was estimated using niche modelling (43) and population dynamics of Aedes aegypti (45) were studied. Studies on optimal control and prevention of ZIKV included the introduction of Aedes aegypti-suppressing Wolbachia mosquitoes (46).
Furthermore, modelling approaches were used to estimate the effectiveness of intervention strategies to control the spread of ZIKV (48,49). A spatial data mining method was applied to predict potential host-virus interaction that identified ten mammals, including bats, as non-human hosts of ZIKV (51). Finally, a lower risk of ZIKV induced microcephaly due to a yellow fever vaccine coverage was highlighted through a causal analysis (50).
Big data
This review identified 19/57 studies that described the use of big data in the context of the Zika outbreak. This included mainly description and analysis of different roles and the use of social media (16/57) and web-based surveillance (n=3/57) during the outbreak. The largest number of studies (n=11/57) undertook Facebook/Twitter analytics (55,57-62,64,65,69) and analysis of obstetric websites and their associated social media accounts (68). Several studies identified, through social media analytics, that platforms such as Facebook and Twitter were important tools during the Zika outbreak. These were used by the national and international health agencies including the WHO and the CDC for the purposes of outreach, health communication, online engagement (62,63) and social media monitoring of public awareness (67). Furthermore, studies looked at social media engagement of either the public (61,64) or both the public and the CDC (60) to study their concerns during the Zika outbreak. Other studies combined data from different web-based data streams such as Google trends, Twitter and HealthMap with traditional surveillance case data to track and predict Zika incidence during the 2016 Latin America outbreak (69). The relationship between daily Zika related news coverage, social media mentions and online behaviour regarding Zika was also studied (58). Two studies applied social media analysis to photo sharing (Instagram and Pinterest) (65) and a video sharing website (YouTube) that found relevant content on Zika (66).
This review also identified studies (4/57) that used social media analytics to investigate the issue of misinformation and pseudo-scientific claims about Zika on these platforms (54-57). It was reported that misinformation about ZIKV existed on the regular search engines such as Google and Bing (54), and they were far more popular than the posts dispersing accurate information (56). In addition, one study identified that obstetric care practices do not optimally use their website to inform populations about the risk of ZIKV (68).
The category of big data also included studies on web-based surveillance (3/57) particularly on OpenZika project, ProMED, and Digital Participatory Surveillance (DPS) (14,52,53). The OpenZika project, an IBM World Community Grid Project, started the virtual screening of approximately 6 million compounds to discover novel candidate compounds for developing new drugs to treat ZIKV infection (52). ProMED is one of the most innovative and important contributors to global health digital surveillance (53). ProMED is an innovative and informal disease surveillance system, which collects and disseminates data on outbreaks faster than traditional surveillance systems (53). Evidence suggests that it was one of the first outlets to report the early spread of Zika in the Americas in 2015 (53). In addition, DPS is another form of digital surveillance where the public reports signs and symptoms through a structured form, which can be aggregated, analysed and summarized via epidemiological maps to enable risk prediction and inform prevention and response (14). Although DPS had strong potential to demonstrate its value during the global Zika outbreak, there is a lack of representation of its use in the literature (14).
mHealth
Four studies (n=4/57) described the use of mHealth applications in the context of the Zika outbreak. Zika tracker is a mobile web-based application that can be used globally to report suspected or confirmed Zika cases and its related signs and symptoms on a public or private level (70).
Another mobile web-based application is the WHO’s Zika App, which provides real-time information to all users (71). The application has both a mobile phone and website interface and has a general section for the public and a more technical section for healthcare workers. The general section provides general disease-related information including key facts, symptoms and transmission, diagnosis and prevention, while the section for health care workers provides more technical information on diagnostics, surveillance, management of complications, vector control, and the WHO Response Plan.
A smartphone-based diagnostic platform for rapid detection of ZIKV showed high sensitivity and specificity in detection of ZIKV (72). The platform employs a smartphone-based algorithm and can be powered by a USB (e.g., a USB power bank) or solar panel. The tool has wide spread clinical deployment as it can detect ZIKV directly from crude human sample matrices (blood, urine, and saliva) (72).
This review also revealed a study testing the utility of a dedicated smartphone application to improve the sensitivity of optical analysis of results obtained from a paper-based device used for the detection of ZIKV (73). Furthermore, the results could be sent directly via SMS to a health center (e.g., laboratory), for example (73).
Novel technologies
This review identified two (n=2/57) studies that described the use of innovative and novel technologies during the Zika outbreak. MinION, an innovative real-time, portable genome-sequencing device was used in Brazil to perform portable whole-genome sequencing after tiling polymerase chain reaction (PCR) (75).
The second of these technologies served the function of prevention and control. Rodriguez et al. (74) demonstrated in their experimental study the efficacy of some wearable insecticide devices compared with traditional repellent for the Aedes aegypti. The study concluded that the wearable device, which releases Metofluthrin, significantly reduced the numbers of attracted mosquitoes (74).
Discussion
The findings suggest that the overall utilization of digital technologies during the Zika outbreak is covered somewhat disproportionately in the scientific literature between January 2016 and July 2017. We identified relatively more articles on the utilization of computational modelling (n=32) and big data (n=19). While we identified only a few studies on mHealth (n=4) and novel technologies (n=2), as also shown in Figure 2. This could be possibly explained by the priority of action over research during a PHEIC.
A large number of the papers focused on the region of the Americas, where the burden of the Zika outbreak was greatest. However, considering the rapid spread of the outbreak to other regions, nearly half of the papers did not target a particular region. The rapidly spreading ZIKV disease was usually mild and not fatal, but it was associated with long-term neurological complications including microcephaly during pregnancy (3). Therefore, one of the important public health priorities was to bring evidence through research in order to prevent and control the transmission of this infection. Indeed, most of the digital technologies identified in this review served the function of monitoring, focusing mainly on prevention and control of the outbreak, whilst only a few digital technologies served the function of diagnostics or treatment.
Digital technologies played an important role in the Zika outbreak, aiding understanding of the disease itself, improving diagnostic tools and controlling the spread of the infection. The most used and documented digital technologies were data modelling and big data analytics. As the Zika outbreak was faced with a lack of adequate research and knowledge on transmission dynamics of ZIKV and its vectors (76), improving knowledge and evidence about ZIKV became an important priority. Data modelling methods along with high-resolution geographical and spatial models were used as powerful tools to understand transmission dynamics and dispersion of ZIKV, and to estimate the burden of the infection in multiple regions. For example, one model-based study (20) estimated that 1.65 (1.5–2.1) million childbearing women and 93.4 (81.6–117.1) million people in total could be infected in Americas as a result of the outbreak. Another modelling study (50) predicted that high yellow fever vaccination coverage among pregnant women in Brazil might expose their offspring to lower risk of developing microcephaly.
Additionally, studies on social media analytics reveal a high value to health communication and engagement during a public health crisis. For example, both the public and health authorities showed high online engagement through social media during the Zika outbreak (62,63), which highlights the potential role of social media as a platform for health information dissemination during an outbreak. However, such approaches could be faced with the challenge of misinformation and circulation of pseudo-scientific claims (54-57) and therefore require better ‘curation’ of health-related posts on these platforms (56), and does not replace the need for other relevant interventions during an outbreak. One solution to address concerns surrounding the reliability of information shared on social media is to use a system such as WHO’s mHealth Zika application, capable of disseminating real-time health information to all of its users (71). Nonetheless, such approaches have far fewer participants than popular social media platforms, limiting their overall efficacy.
The use of other digital technologies identified in this review, including but not confined to Zika tracker (70) and DPS (14), demonstrate their potential to contribute to the monitoring of disease outbreaks and public health. Information obtained through such digital applications could be used by a variety of public health workers (e.g., bioinformaticians and epidemiologists) to understand ZIKV disease and target preventive and control measures in the target areas (70). Digital technologies such as OpenZika could accelerate the discovery of new antivirals against Zika (52). However, it is important to note that there are limited research and evidence on the effectiveness of certain digital technologies in the context of the Zika outbreak. For example, there is limited evidence in the published literature on substantive public health outcomes for the DPS projects during the outbreak, possibly reflecting the priority of action over research during a PHEIC (14), as mentioned earlier. Moreover, most of the identified published papers in this review used non-experimental methods, therefore further quantitative experimental research could add more evidence for the effectiveness of several additional novel technologies, particularly mHealth approaches and novel technologies with reference to PHEIC.
The scope of this review has potential limitations relating to the search strategy that should be considered when interpreting the results. Only two databases were used for the literature search, thus some articles present only in other databases will have been missed. Furthermore, only articles in the English language were included in the review, however, only two articles in other languages (Spanish and Chinese) identified in this review were excluded from the final synthesis due to this criterion.
Conclusions
This scoping review summarized the breadth of research activity on the utilization of digital technologies during the 2016 Zika outbreak. The review revealed the use of a number of digital technologies with the majority of publications reporting the use of computational modelling (including GIS and spatial analysis) and big data systems as core approaches, commonly dedicated to disease monitoring. Only a very small share of these studies described the use of mHealth and novel technologies which were dedicated not only to monitoring, but also to improving point-of-care diagnostic tools. Considering the potential for rapid implementation of this form of technologies or smartphone applications, they warrant further study. Our findings suggest that digital technologies could offer significant improvements for disease monitoring, enhanced diagnostic tools, and prevention and control of disease spread, provided these approaches are evidence-based and have measurable impacts to support continued development.
Supplementary
Table S1
| Database | Search Syntax |
|---|---|
| PubMed | (("Zika"[Title/Abstract] OR "Zika virus infection"[Title/Abstract] OR "ZIKV"[Title/Abstract] OR "Zika virus disease"[Title/Abstract] OR "viral disease"[Title/Abstract]) AND ("Digital"[Title/Abstract] OR "Technology"[Title/Abstract] OR "Precision medicine"[Title/Abstract] OR "Biosensor"[Title/Abstract] OR "Sensors"[Title/Abstract] OR "Bio-surveillance"[Title/Abstract] OR "Intelligent surveillance"[Title/Abstract] OR "Participatory surveillance"[Title/Abstract] OR "Genomic epidemiology"[Title/Abstract] OR "Genomic sequencing"[Title/Abstract] OR "Pathogen genomics"[Title/Abstract] OR "Big data"[Title/Abstract] OR "Data storage"[Title/Abstract] OR "Data science"[Title/Abstract] OR "Information processing"[Title/Abstract] OR "Blockchain"[Title/Abstract] OR "Social media"[Title/Abstract] OR "Twitter"[Title/Abstract] OR "Facebook"[Title/Abstract] OR "Instagram"[Title/Abstract] OR "Flicker"[Title/Abstract] OR "YouTube"[Title/Abstract] OR "Wikipedia"[Title/Abstract] OR "Telemedicine"[Title/Abstract] OR "Robotics"[Title/Abstract] OR "Machine learning"[Title/Abstract] OR "Modelling"[Title/Abstract] OR "Mathematical modelling"[Title/Abstract] OR "Spatiotemporal modelling"[Title/Abstract] OR "Mapping"[Title/Abstract] OR "mHealth"[Title/Abstract] OR "Mobilephone"[Title/Abstract] OR "Smartphone"[Title/Abstract] OR "Cellphone"[Title/Abstract] OR "Phone"[Title/Abstract] OR "Cell phone technology"[Title/Abstract] OR "Mobile data"[Title/Abstract] OR "Mobile application"[Title/Abstract] OR "Devices"[Title/Abstract] OR "Connected device"[Title/Abstract] OR "Internet"[Title/Abstract] OR "Web-based"[Title/Abstract] OR "Internet-based"[Title/Abstract] OR "Web-database"[Title/Abstract] OR "Cloud"[Title/Abstract] OR "Cloud-based"[Title/Abstract] OR "eHealth"[Title/Abstract] OR "E-learning"[Title/Abstract] OR "Game-based learning"[Title/Abstract] OR "Augmented reality"[Title/Abstract] OR "Massive Online Open Courses"[Title/Abstract] OR "MOOC"[Title/Abstract] OR "Virtual learning"[Title/Abstract] OR "Virtual reality"[Title/Abstract] OR "Online learning"[Title/Abstract] OR "Gaming technology"[Title/Abstract] OR "Serious game"[Title/Abstract] OR "Crowd sourcing"[Title/Abstract] OR "Citizen Science"[Title/Abstract] OR "Connected device"[Title/Abstract] OR "Remote-sensing technology"[Title/Abstract] OR "Satellite"[Title/Abstract] OR "GPS"[Title/Abstract] OR "Global Positioning System"[Title/Abstract] OR "Geographic Information System"[Title/Abstract] OR "GIS"[Title/Abstract] OR "Spatial"[Title/Abstract] OR "Participatory"[Title/Abstract] OR "Sensor"[Title/Abstract] OR "App"[Title/Abstract] OR "Artificial intelligence"[Title/Abstract] OR "Tracking"[Title/Abstract] OR "Mapping"[Title/Abstract] OR "Biogeography"[Title/Abstract] OR "Biomarkers"[Title/Abstract] OR "Disease mapping"[Title/Abstract])) AND ("2015/01/01"[PDAT]: "2017/07/23"[PDAT]) |
| Web of Science | TITLE:("Zika"OR "Zika virus infection"OR "ZIKV" OR "Zika virus disease" OR "Viral disease") AND TITLE: (Digital OR Technology OR Precision medicine OR Biosensor OR Sensors OR Bio-surveillance OR Intelligent surveillance OR Participatory surveillance OR Genomic epidemiology OR Genomic sequencing OR Pathogen genomics OR Big data OR Data storage OR Data science OR Information processing OR Blockchain OR Social media OR Twitter OR Facebook OR Instagram OR Flicker OR YouTube OR Wikipedia OR Telemedicine OR Robotics OR Machine learning OR Modelling OR Mathematical modelling OR Spatiotemporal modelling OR Mapping OR mHealth OR Mobilephone OR Smartphone OR Cellphone OR Phone OR Cell phone technology OR Mobile data OR "Mobile application" OR "Devices" OR "Connected device" OR "Internet" OR "Web-based" OR "Internet-based" OR "Web-database" OR "Cloud" OR "Cloud-based" OR "eHealth" OR "E-learning" OR "Game-based learning" OR "Augmented reality" OR "Massive Online Open Courses" OR "MOOC" OR "Virtual learning" OR "Virtual reality" OR "Online learning" OR "Gaming technology" OR "Serious game" OR "Crowd sourcing" OR "Citizen Science" OR "Connected device" OR "Remote-sensing technology" OR "Satellite" OR "GPS" OR "Global Positioning System" OR "Geographic Information System" OR Drones OR "GIS" OR "Spatial" OR "Participatory" OR "Sensor" OR "App" OR "Artificial intelligence" OR "Tracking" OR "Mapping" OR "Biogeography" OR "Biomarkers" OR "Disease mapping") |
Acknowledgments
Funding: This work was supported by the Institute of Global Health, Faculty of Medicine, University of Geneva.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Antoine Flahault and Olga De Santis) for the series “Precision Infectious Disease Epidemiology” published in Journal of Public Health and Emergency. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2018.05.02). The series “Precision Infectious Disease Epidemiology” was commissioned by the editorial office without any funding or sponsorship. AF serves as an unpaid editorial board member of Journal of Public Health and Emergency from Apr 2018 to Mar 2020 and served as the unpaid Guest Editor of the series. ODS served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016;387:1531-9. [Crossref] [PubMed]
- Tambo E, Kazienga A, Talla M, et al. Digital Technology and Mobile Applications Impact on Zika and Ebola Epidemics Data Sharing and Emergency Response. Journal of Health & Medical Informatics 2017;8:254.
- World Health Organization. Zika virus. WHO. 2016. Available online: http://www.who.int/mediacentre/factsheets/zika/en/
- Krow-Lucal ER, Biggerstaff BJ, Staples JE. Estimated Incubation Period for Zika Virus Disease. Emerg Infect Dis 2017;23:841-5. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Zika Virus. Centers for Disease Control and Prevention. 2017. Available online: https://www.cdc.gov/zika/about/overview.html
- Baud D, Gubler DJ, Schaub B, et al. An update on Zika virus infection. Lancet 2017;390:2099-109. [Crossref] [PubMed]
- World Health Organization. Zika virus outbreak global response. Interim report. Geneva, Switzerland: World Health Organization 2016 27/05/2016. Report No.: WHO/ZIKV/SRF/16.2.
- Lucey DR. Time for global action on Zika virus epidemic. BMJ 2016;352:i781. [Crossref] [PubMed]
- Korzeniewski K, Juszczak D, Zwolinska E. Zika - another threat on the epidemiological map of the world. Int Marit Health 2016;67:31-7. [Crossref] [PubMed]
- Pan American Health Organization/World Health Organistion. Zika - Epidemiological Report Brazil. Washington, D.C: PAHO/WHO2017.
- Samarasekera U, Triunfol M. Concern over Zika virus grips the world. Lancet 2016;387:521-4. [Crossref] [PubMed]
- Dutra HL, Rocha MN, Dias FB, et al. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe 2016;19:771-4. [Crossref] [PubMed]
- Ferguson NM, Kien DT, Clapham H, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 2015;7:279ra37 [Crossref] [PubMed]
- Pagliari C, Vijaykumar S. Digital Participatory Surveillance and the Zika Crisis: Opportunities and Caveats. PLoS Negl Trop Dis 2016;10:e0004795 [Crossref] [PubMed]
- Chan K, Weaver SC, Wong PY, et al. Rapid, Affordable and Portable Medium-Throughput Molecular Device for Zika Virus. Sci Rep 2016;6:38223. [Crossref] [PubMed]
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. International journal of social research methodology 2005;8:19-32. [Crossref]
- Squires H, Tappenden P. Mathematical modelling and its application to social care. 2011. Available online: http://eprints.lse.ac.uk/id/eprint/41192
- Ward JS, Barker A. Undefined By Data: A Survey of Big Data Definitions. arXiv:1309.5821, 2013.
- WHO Global Observatory for eHealth. mHealth: New horizons for health through mobile technologies. Geneva: World Health Organization, 2011.
- Alex Perkins T, Siraj AS, Ruktanonchai CW, et al. Model-based projections of Zika virus infections in childbearing women in the Americas. Nat Microbiol 2016;1:16126. [Crossref] [PubMed]
- Rodriguez-Morales AJ, Patino-Cadavid LJ, Lozada-Riascos CO, et al. Mapping Zika in municipalities of one coastal department of Colombia (Sucre) using geographic information systems during the 2015-2016 outbreak: implications for public health and travel advice. Int J Infect Dis 2016;48:70-2. [Crossref] [PubMed]
- Rodriguez-Morales AJ, Galindo-Marquez ML, Garcia-Loaiza CJ, et al. Mapping Zika virus infection using geographical information systems in Tolima, Colombia, 2015-2016. F1000Res 2016;5:568. [Crossref] [PubMed]
- Rodriguez-Morales AJ, Ruiz P, Tabares J, et al. Mapping the ecoepidemiology of Zika virus infection in urban and rural areas of Pereira, Risaralda, Colombia, 2015-2016: Implications for public health and travel medicine. Travel Med Infect Dis 2017;18:57-66. [Crossref] [PubMed]
- Rodriguez-Morales AJ, Galindo-Marquez ML, Garcia-Loaiza CJ, et al. Mapping Zika virus disease incidence in Valle del Cauca. Infection 2017;45:93-102. [Crossref] [PubMed]
- Rodriguez-Morales AJ, Garcia-Loaiza CJ, Galindo-Marquez ML, et al. Zika infection GIS-based mapping suggest high transmission activity in the border area of La Guajira, Colombia, a northeastern coast Caribbean department, 2015-2016: Implications for public health, migration and travel. Travel Med Infect Dis 2016;14:286-8. [Crossref] [PubMed]
- Messina JP, Kraemer MU, Brady OJ, et al. Mapping global environmental suitability for Zika virus. Elife 2016;5:e15272 [Crossref] [PubMed]
- Majumder MS, Santillana M, Mekaru SR, et al. Utilizing Nontraditional Data Sources for Near Real-Time Estimation of Transmission Dynamics During the 2015-2016 Colombian Zika Virus Disease Outbreak. JMIR Public Health Surveill 2016;2:e30 [Crossref] [PubMed]
- Kucharski AJ, Funk S, Eggo RM, et al. Transmission Dynamics of Zika Virus in Island Populations: A Modelling Analysis of the 2013-14 French Polynesia Outbreak. PLoS Negl Trop Dis 2016;10:e0004726 [Crossref] [PubMed]
- Gao D, Lou Y, He D, et al. Prevention and Control of Zika as a Mosquito-Borne and Sexually Transmitted Disease: A Mathematical Modeling Analysis. Sci Rep 2016;6:28070. [Crossref] [PubMed]
- Bogoch II, Brady OJ, Kraemer MUG, et al. Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: a modelling study. Lancet Infect Dis 2016;16:1237-45. [Crossref] [PubMed]
- Mordecai EA, Cohen JM, Evans MV, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis 2017;11:e0005568 [Crossref] [PubMed]
- Castro LA, Fox SJ, Chen X, et al. Assessing real-time Zika risk in the United States. BMC Infect Dis 2017;17:284. [Crossref] [PubMed]
- Caminade C, Turner J, Metelmann S, et al. Global risk model for vector-borne transmission of Zika virus reveals the role of El Nino 2015. Proc Natl Acad Sci U S A 2017;114:119-24. [Crossref] [PubMed]
- Agusto FB, Bewick S, Fagan WF. Mathematical model for Zika virus dynamics with sexual transmission route. Ecological Complexity 2017;29:61-81. [Crossref]
- Kumar N, Abdullah M, Faizan MI, et al. Progression dynamics of Zika fever outbreak in El Salvador during 2015–2016: a mathematical modeling approach. Future Virology 2017;12:271-81. [Crossref]
- Fitzgibbon WE, Morgan JJ, Webb GF. An outbreak vector-host epidemic model with spatial structure: the 2015-2016 Zika outbreak in Rio De Janeiro. Theor Biol Med Model 2017;14:7. [Crossref] [PubMed]
- Manrique PD, Xu C, Hui PM, et al. Atypical viral dynamics from transport through popular places. Phys Rev E 2016;94:022304 [Crossref] [PubMed]
- Samy AM, Thomas SM, Wahed AA, et al. Mapping the global geographic potential of Zika virus spread. Mem Inst Oswaldo Cruz 2016;111:559-60. [Crossref] [PubMed]
- Massad E, Tan SH, Khan K, et al. Estimated Zika virus importations to Europe by travellers from Brazil. Glob Health Action 2016;9:31669. [Crossref] [PubMed]
- Zinszer K, Morrison K, Brownstein JS, et al. Reconstruction of Zika Virus Introduction in Brazil. Emerg Infect Dis 2017;23:91-4. [Crossref] [PubMed]
- Zhang Q, Sun K, Chinazzi M, et al. Spread of Zika virus in the Americas. Proc Natl Acad Sci U S A 2017;114:E4334-E43. [Crossref] [PubMed]
- Attaway DF, Waters NM, Geraghty EM, et al. Zika virus: Endemic and epidemic ranges of Aedes mosquito transmission. J Infect Public Health 2017;10:120-3. [Crossref] [PubMed]
- Alaniz AJ, Bacigalupo A, Cattan PE. Spatial quantification of the world population potentially exposed to Zika virus. Int J Epidemiol 2017;46:966-75. [Crossref] [PubMed]
- Santos J, Meneses BM. An integrated approach for the assessment of the Aedes aegypti and Aedes albopictus global spatial distribution, and determination of the zones susceptible to the development of Zika virus. Acta Trop 2017;168:80-90. [Crossref] [PubMed]
- Legros M, Otero M, Aznar VR, et al. Comparison of Two Detailed Models of Aedes aegypti Population Dynamics. Ecosphere 2016;7:e01515 [Crossref] [PubMed]
- Turelli M, Barton NH. Deploying dengue-suppressing Wolbachia: Robust models predict slow but effective spatial spread in Aedes aegypti. Theor Popul Biol 2017;115:45-60. [Crossref] [PubMed]
- Evans MV, Dallas TA, Han BA, et al. Data-driven identification of potential Zika virus vectors. Elife 2017;6:e22053 [Crossref] [PubMed]
- Lee EK, Liu Y, Pietz FH. A Compartmental Model for Zika Virus with Dynamic Human and Vector Populations. AMIA Annu Symp Proc 2016;2016:743-52.
- Ding CX, Tao NN, Zhu YG. A Mathematical Model of Zika virus and its Optimal Control. 2016. Available online: https://ieeexplore.ieee.org/abstract/document/7553763/metrics
- De Goes Cavalcanti LP, Tauil PL, Alencar CH, et al. Zika virus infection, associated microcephaly, and low yellow fever vaccination coverage in Brazil: is there any causal link? J Infect Dev Ctries 2016;10:563-6. [Crossref] [PubMed]
- Gonzalez-Salazar C, Stephens CR, Sanchez-Cordero V. Predicting the Potential Role of Non-human Hosts in Zika Virus Maintenance. Ecohealth 2017;14:171-7. [Crossref] [PubMed]
- Ekins S, Perryman AL, Horta Andrade C. OpenZika: An IBM World Community Grid Project to Accelerate Zika Virus Drug Discovery. PLoS Negl Trop Dis 2016;10:e0005023 [Crossref] [PubMed]
- Carrion M, Madoff LC. ProMED-mail: 22 years of digital surveillance of emerging infectious diseases. Int Health 2017;9:177-83. [Crossref] [PubMed]
- Venkatraman A, Mukhija D, Kumar N, et al. Zika virus misinformation on the internet. Travel Med Infect Dis 2016;14:421-2. [Crossref] [PubMed]
- Dredze M, Broniatowski DA, Hilyard KM. Zika vaccine misconceptions: A social media analysis. Vaccine 2016;34:3441-2. [Crossref] [PubMed]
- Sharma M, Yadav K, Yadav N, et al. Zika virus pandemic-analysis of Facebook as a social media health information platform. Am J Infect Control 2017;45:301-2. [Crossref] [PubMed]
- Bode L, Vraga EK. See Something, Say Something: Correction of Global Health Misinformation on Social Media. Health Commun 2018;33:1131-40. [PubMed]
- Southwell BG, Dolina S, Jimenez-Magdaleno K, et al. Zika Virus-Related News Coverage and Online Behavior, United States, Guatemala, and Brazil. Emerg Infect Dis 2016;22:1320-1. [Crossref] [PubMed]
- Khatua A, Khatua A. Immediate and Long-term Effects of 2016 Zika Outbreak: A Twitter-based Study. 2016 IEEE 18th International Conference on E-Health Networking, Applications and Services (Healthcom) 2016:436-41.
- Glowacki EM, Lazard AJ, Wilcox GB, et al. Identifying the public's concerns and the Centers for Disease Control and Prevention's reactions during a health crisis: An analysis of a Zika live Twitter chat. Am J Infect Control 2016;44:1709-11. [Crossref] [PubMed]
- Fu KW, Liang H, Saroha N, et al. How people react to Zika virus outbreaks on Twitter? A computational content analysis. Am J Infect Control 2016;44:1700-2. [Crossref] [PubMed]
- Vijaykumar S, Meurzec RW, Jayasundar K, et al. What's buzzing on your feed? Health authorities' use of Facebook to combat Zika in Singapore. J Am Med Inform Assoc 2017;24:1155-9. [Crossref] [PubMed]
- Stefanidis A, Vraga E, Lamprianidis G, et al. Zika in Twitter: Temporal Variations of Locations, Actors, and Concepts. JMIR Public Health Surveill 2017;3:e22 [Crossref] [PubMed]
- Miller M, Banerjee T, Muppalla R, et al. What Are People Tweeting About Zika? An Exploratory Study Concerning Its Symptoms, Treatment, Transmission, and Prevention. JMIR Public Health Surveill 2017;3:e38 [Crossref] [PubMed]
- Fung IC, Blankenship EB, Goff ME, et al. Zika-Virus-Related Photo Sharing on Pinterest and Instagram. Disaster Med Public Health Prep 2017;11:656-9. [Crossref] [PubMed]
- Basch CH, Fung IC, Hammond RN, et al. Zika Virus on YouTube: An Analysis of English-language Video Content by Source. J Prev Med Public Health 2017;50:133-40. [Crossref] [PubMed]
- Avery EJ. Public information officers’ social media monitoring during the Zika virus crisis, a global health threat surrounded by public uncertainty. Public Relations Review 2017;43:468-76. [Crossref]
- Lehnert JD, Ellingson MK, Goryoka GW, et al. Use of Obstetric Practice Web Sites to Distribute Zika Virus Information to Pregnant Women During a Zika Virus Outbreak. J Public Health Manag Pract 2017;23:608-13. [Crossref] [PubMed]
- McGough SF, Brownstein JS, Hawkins JB, et al. Forecasting Zika Incidence in the 2016 Latin America Outbreak Combining Traditional Disease Surveillance with Search, Social Media, and News Report Data. PLoS Negl Trop Dis 2017;11:e0005295 [Crossref] [PubMed]
- Kelvin AA, Banner D, Pamplona L, et al. ZIKATracker: A mobile App for reporting cases of ZIKV worldwide. J Infect Dev Ctries 2016;10:113-5. [Crossref] [PubMed]
- Chiodini J. ZIKA App - A great resource from the World Health Organization (WHO). Travel Med Infect Dis 2016;14:539-40. [Crossref] [PubMed]
- Priye A, Bird SW, Light YK, et al. A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci Rep 2017;7:44778. [Crossref] [PubMed]
- Bedin F, Boulet L, Voilin E, et al. Paper-based point-of-care testing for cost-effective diagnosis of acute flavivirus infections. J Med Virol 2017;89:1520-7. [Crossref] [PubMed]
- Rodriguez SD, Chung HN, Gonzales KK, et al. Efficacy of Some Wearable Devices Compared with Spray-On Insect Repellents for the Yellow Fever Mosquito, Aedes aegypti (L.) (Diptera: Culicidae). J Insect Sci 2017;17: [Crossref] [PubMed]
- Faria NR, Sabino EC, Nunes MR, et al. Mobile real-time surveillance of Zika virus in Brazil. Genome Med 2016;8:97. [Crossref] [PubMed]
- Perkins TA. Retracing Zika's footsteps across the Americas with computational modeling. Proc Natl Acad Sci U S A 2017;114:5558-60. [Crossref] [PubMed]
Cite this article as: Ahmadi S, Bempong NE, De Santis O, Sheath D, Flahault A. The role of digital technologies in tackling the Zika outbreak: a scoping review. J Public Health Emerg 2018;2:20.