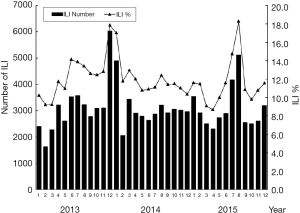
Influenza surveillance in Jiangyin from 2013 to 2015
Introduction
Seasonal influenza is an acute viral infection caused by an influenza virus. When an infected person coughs, infected droplets get into the air and another person can breathe them in and be exposed (1). Influenza viruses are enveloped viruses of the family Orthomyxoviridae that contain a segmented RNA genome. The long-term epidemiologic success of influenza viruses is primarily due to antigenic variation that takes place in the two surface glycoproteins of the virus, the HA and NA. Of the three types of influenza viruses, A, B, and C, the first two are associated with significant seasonal morbidity and mortality (2,3).
Influenza is a major health threat throughout the world, which occurs globally with an annual attack rate estimated at 5–10% in adults and 20–30% in children. Illnesses can result in hospitalization and death mainly among high-risk groups (the very young, elderly or chronically ill). These annual epidemics are estimated to result in about 3 to 5 million cases of severe illness, and about 250,000 to 500,000 deaths worldwide (4). The World Health Organization (WHO) is urging intensification of influenza surveillance as a part of contingency planning for responses to influenza pandemics. Thus, the epidemiology and prevalence of influenza have been the topic of extensive investigation (5).
Hospital based influenza-like illness (ILI) surveillance is essentially a type of syndromic surveillance (6). Jiangyin People’s Hospital and Jiangyin Shanguan Hospital as influenza surveillance sentinel hospitals have been included in Wuxi influenza surveillance network since May 2012. They mainly undertake the work of influenza-like cases report and sampling, meanwhile detection of pathogenic influenza was undertaken by center for disease control and prevention of Jiangyin. Influenza was monitored in the region from 2013 to 2015, and the result was analyzed, in order to understand the epidemiological characteristics of influenza in this region, and provide scientific basis for prevention and control.
Methods
Study subjects
In the absence of laboratory confirmation, potential influenza cases can be identified with a clinical definition of influenza, ILI. Influenza was detected by virus isolation from nasopharyngeal swabs of ILI patients who reported to medical clinic, pediatric department, the emergency department and outpatient clinics in two influenza surveillance sentinel hospital between 2013 and 2015.
Influenza surveillance systems
Influenza surveillance included both sentinel hospital ILI surveillance and laboratory-confirmed surveillance.
Sentinel hospital influenza-like illness (ILI) surveillance
According to the guideline for case definition of ILI (7), patients presenting with fever (≥38 °C), cough and/or a sore throat at any of two sentinel hospitals were counted by physicians as ILI cases for sentinel hospital ILI surveillance. The number of ILI cases and total number of clinical consultations were collected from medical clinic, pediatrics, and emergency room departments, and recorded daily. The ILI rate was defined as the number of ILI cases out of the total number of consultations, expressed as a percentage (ILI %), and calculated for each calendar week of this study. ILI % usually can reflect the level of the ILI within a certain time in influenza surveillance.
Laboratory-confirmed surveillance
Throat swab specimens were collected from ILI patients of two sentinel hospitals. Each sentinel hospital was required to collect 5–10 specimens from suspect ILI patients weekly, Specimens were transported to the CDC laboratory in a suitable transport medium at 4 °C within 48 h of collection for the laboratory confirmation and subtype identification. The influenza genome was amplified and detected using a standardized the real-time reverse transcription (rRT)-PCR assay, to test for influenza A and/or B types. Samples positive for influenza A and/or B were further tested to confirm influenza A and/or B positivity and to determine influenza A subtypes. Specimens testing positive for influenza by rRT-PCR were inoculated onto Madin-Darby canine kidney (MDCK) cells for isolation of influenza strains. The influenza strains were analyzed by a hemagglutination inhibition test using reference antigens and anti-sera.
Statistical analysis
Database was built by Excel 2007 software, R3.2.0 software was applied for statistical analysis. Histogram and line charts were used to show the time distribution of ILI cases. The χ2 test or fisher exact test was used to assess differences in proportion. A two-sided P value of <0.05 was considered significant different.
Results
Characteristic of enrolled patients
A total of 927,656 cases were enrolled from two sentinel hospitals from 2013 to 2015, of which 111,749 were ILI, the ILI % was 12.05%. The number of enrolled ILI cases gradually increased during the study period, but the ILI % was highest in 2013 (12.54%), and showed a trend of decreasing year by year (Table 1).
Table 1
| Year | ILI case | Total case | ILI % |
|---|---|---|---|
| 2013 | 37,012 | 295,220 | 12.54 |
| 2014 | 37,055 | 306,868 | 12.08 |
| 2015 | 37,682 | 325,568 | 11.57 |
| Totally | 111,749 | 927,656 | 12.05 |
ILI, influenza-like illness.
Seasonality of influenza-like illness (ILI)
The peak time of ILI cases and epidemic duration are presented in Figure 1. There were two obvious seasonal peaks of ILI each year. Cases were mostly reported during the cold and dry seasons, from December to February in winter and from June to August in summer. In 2013, the major peak appeared in December, while the peaks appeared in January and August in 2014 as well as in 2015.
Age distribution of influenza-like illness (ILI)
The patient’s age ranged between 2 days and 80 years. The age composition of ILI from 2013 to 2015 was showed in Table 2. Children aged <5 years accounted for 55.27% (65,119/111,749) of the total number of ILI, which is the highest among age groups. The number of ILI cases in this group gradually increased during the three years. Age group of 5–14 years old accounted for second (18.71%) of the total number of ILI. Individuals >60 years of age accounted for 4.05% of the ILI, whose number of cases was the least. There was a statistically significant difference in the proportion of age enrolled among ILI cases (χ2=559.43, P<0.001).
Table 2
| Year | Age group (%) | ||||
|---|---|---|---|---|---|
| 0- | 5- | 15- | 25- | 60- | |
| 2013 | 20,990 (56.71) | 7,968 (21.53) | 1,845 (4.98) | 5,052 (13.65) | 1,157 (3.13) |
| 2014 | 21,608 (58.31) | 6,988 (18.86) | 1,553 (4.19) | 5,142 (13.88) | 1,764 (4.76) |
| 2015 | 22,521 (59.77) | 5,949 (15.79) | 1,841 (4.89) | 5,765 (15.30) | 1,606 (4.26) |
| Totally | 65,119 (55.27) | 20,905 (18.71) | 5,239 (4.69) | 15,959 (14.28) | 4,527 (4.05) |
ILI, influenza-like illness.
Detection of influenza virus
Laboratory results were obtained from 1,591 ILI cases from 2013 to 2015, of which 247 (15.52%) tested positive for influenza virus. The strain distribution of influenza from 2013 to 2015 was shown in Table 3. Type A (H3) and type B were the main influenza strains in the region. Of the 247 influenza positive cases 132 (53.44%) were A (H3), 26 (10.53%) were A (H1N1) and 85 (34.41%) were B. The proportion of samples testing positive for influenza viruses was highest in 2014 (20.30%) among ILI patients and followed by 2015 (17.33%). A (H3) was the major influenza strain of 2013, accounting for 85.11%. While, A (H3) as well as type B were major influenza strains of 2014 and 2015. The strain structure of positive cases was significant different among different years (Fisher’s Exact Test, P<0.001).
Table 3
| Year | Number of samples | Influenza strain | Positive number | Positive rate (%) | |||
|---|---|---|---|---|---|---|---|
| A (H3) | A (H1N1) | A (unclassified) | B | ||||
| 2013 | 528 | 40 | 7 | 0 | 0 | 47 | 8.90 |
| 2014 | 532 | 47 | 17 | 4 | 40 | 108 | 20.30 |
| 2015 | 531 | 45 | 2 | 0 | 45 | 92 | 17.33 |
| Totally | 1,591 | 132 | 26 | 4 | 85 | 247 | 15.52 |
Seasonality of influenza virus and the correlation with influenza-like illness (ILI) %
Influenza virus was detected predominantly from 2013 to 2015, with peak viral activity observed in December of 2013, January and August of 2014, January and August of 2015, which corresponds to the cold and dry seasons (Figure 2). According to the bar charts showing influenza subtypes (Figure 2), the proportion of influenza virus subtypes changed frequently between 2013 and 2015. Influenza A (H3) was the dominant circulating subtype in December 2013 and influenza dry seasons (July to September) of 2014 and 2015. Influenza B was first detected in January 2014 and became the dominant subtype from February to April during the 2014–2015 influenza cold seasons.
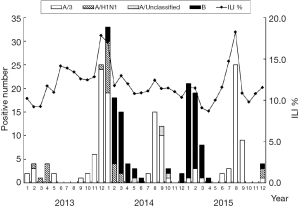
The peak time of positive influenza cases were correspond to the peak time of ILI cases every year, The etiology detection positive rate was positively correlated with ILI % (r=0.603, P=0.0001) (Figure 2).
Discussion
Influenza is a widespread viral infectious disease causing seasonal epidemics and periodic pandemics over the world (8). A better understanding of influenza seasonality and predominant subtypes of influenza virus can help to improve evidence-based prevention and control strategies for influenza in the future.
In this study, we have documented a substantial amount of illness and the seasonal variation of ILI. Moreover, we systematically reviewed influenza activity in Jiangyin by combining epidemiological data and laboratory results.
A high level of ILI was found from 2013 to 2015. There were two obvious seasonal peaks of ILI in winter and summer each year, which accorded with the epidemic characteristics of influenza in southern China (9). The double peak epidemic mode may relate to climate factors in the region. Experimental studies demonstrated that influenza virus survival and transmissibility was dependent on humidity and temperature (10,11). It remains unclear precisely why influenza virus transmission is most efficient under cold, dry conditions, possible mechanism may that virus half-life was increased at lower temperatures and influenza virus was more stable within the nasal passages when the epithelial surface is cooled by colder ambient air (12). Thus our study suggested that surveillance should be strengthened in influenza season to prevent influenza outbreak and studies in future are necessarily to explore the correlation between meteorological factors and influenza activity.
ILI cases was highest among children (1–14 years), especially in children aged <5 years, which was consistent with what has been found in other studies (13,14). Possible reasons may as follows: firstly, besides influenza viruses, respiratory syncytial virus (RSV) contribute to ILI estimates, it is well established that RSV is most common in children <5 years of age (15). Secondly, the varying proportion of ILI cases across age group could be somehow explained. Children aged <5 years were more inclined to hospital, so the ILI cases were more likely to be discovered. Moreover, several researches showed that young age was strong risk factor for ILI. The burden of influenza was highest among children worldwide (14). This may related to the weak immunity of the organism, children may have an altered balance of memory CD4+ T cells, which potentially affects long term CD4+ T cell responses to the influenza infection (16). Therefore, influenza vaccination for children, students and other high risk groups is an effective way of protection (17).
Our study displayed the temporal conformance between etiology detection and ILI cases, suggesting that ILI temporal trends can reflect the actual influenza activity in epidemic seasons. We found that the proportion of influenza virus subtypes changed frequently between 2013 and 2015. It is difficult to carry out influenza virus surveillance. ILI surveillance was sensitive and affected easily by some known and unknown factors, so if ILI cases increased suddenly outside regular epidemic months, it may be a signal of a novel influenza coming (18). Thus, reinforcing surveillance of ILI in assembly occupancies was necessary to prevent an outbreak of influenza.
There were certain limitations in our study, Firstly, we established sentinel surveillance only in two hospitals of the city, and this could potentially affect the generalizability of our results to the entire population. Moreover, our study mainly according to ILI surveillance, which cannot detect non-influenza respiratory pathogens, such as respiratory syncytial virus, adenovirus, and rhinovirus, these viruses can present similarly to influenza (19), which may lead to lower correlations with laboratory-confirmed data. So, a more specific clinical definition should be used to increase the likelihood that ILI cases are influenza.
In conclusion, influenza was a kind of common respiratory infection with obvious seasonality. ILI temporal trends can reflect the actual influenza activity in epidemic seasons, so ILI surveillance has important guiding significance of influenza outbreak. However, timely influenza vaccination for high risk groups is still the most economic and effective means of prevention currently.
Acknowledgments
The authors thank all of the participants.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2016.12.02). XC serves as an unpaid editorial board member of Journal of Public Health and Emergency from Jan 2017 to Dec 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kidd M. Influenza viruses: update on epidemiology, clinical features, treatment and vaccination. Curr Opin Pulm Med 2014;20:242-6. [Crossref] [PubMed]
- Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu Rev Med 2000;51:407-21. [Crossref] [PubMed]
- Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med 1994;180:1273-82. [Crossref] [PubMed]
- World Health Organization. Influenza (Seasonal), March 2014. Available online: http://www.who.int/mediacentre/factsheets/fs211/en/
- WHO. About the Global Influenza Programme. 2011.
- Henning KJ. What is syndromic surveillance? MMWR Suppl 2004;53:5-11. [PubMed]
- Puig-Barberà J, Tormos A, Trushakova S, et al. The Global Influenza Hospital Surveillance Network (GIHSN): A new platform to describe the epidemiology of severe influenza. Influenza Other Respir Viruses 2015. [Epub ahead of print].
- World Health Organization. WHO surveillance case definitions for ILI and SARI. Influenza, 2015.
- Deng F, Luo PF, Qi X, et al. Surveillance and the epidemiological characteristics of influenza in Jiangsu Province from 2009 to 2011. Modern Preventive Medicine 2013;40:2384-8.
- Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol 2002;122:183-91. [Crossref] [PubMed]
- Viboud C, Pakdaman K, Boëlle PY, et al. Association of influenza epidemics with global climate variability. Eur J Epidemiol 2004;19:1055-9. [Crossref] [PubMed]
- Tamerius J, Nelson MI, Zhou SZ, et al. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect 2011;119:439-45. [Crossref] [PubMed]
- Gordon A, Ortega O, Kuan G, et al. Prevalence and seasonality of influenza-like illness in children, Nicaragua, 2005-2007. Emerg Infect Dis 2009;15:408-14. [Crossref] [PubMed]
- Wang D, Zhang T, Wu J, et al. Socio-economic burden of influenza among children younger than 5 years in the outpatient setting in Suzhou, China. PLoS One 2013;8:e69035 [Crossref] [PubMed]
- Malkin E, Yogev R, Abughali N, et al. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One 2013;8:e77104 [Crossref] [PubMed]
- Kang I, Hong MS, Nolasco H, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol 2004;173:673-81. [Crossref] [PubMed]
- King JC Jr, Stoddard JJ, Gaglani MJ, et al. Effectiveness of school-based influenza vaccination. N Engl J Med 2006;355:2523-32. [Crossref] [PubMed]
- De Donno A, Quattrocchi M, Errico P, et al. Epidemiological and virological assessment of influenza activity in Apulia, Italy, during the seasons 2004 - 2005 and 2005 - 2006. J Int Med Res 2007;35:657-65. [Crossref] [PubMed]
- Li H, Wei Q, Tan A, et al. Epidemiological analysis of respiratory viral etiology for influenza-like illness during 2010 in Zhuhai, China. Virol J 2013;10:143. [Crossref] [PubMed]
Cite this article as: Qian C, Chen X, Cao H, Gu M, Ma Y, Yao J, Shu F. Influenza surveillance in Jiangyin from 2013 to 2015. J Public Health Emerg 2017;1:3.