Social behavior is regulated by the adaptive immune system
In our daily life we expect everybody to have correct social behavior. However, some people are suffering from disorders, such as autism spectrum disorder (ASD), which lead to certain abnormalities in social behavior. Individuals with ASD are characterized by deficits in social interactions and communication as well as by repetitive, stereotyped behavior. These behavioral abnormalities are detectable in early childhood and continue throughout life. Although the prevalence of ASD is relatively high, i.e., at least 1 in 68 children in the United States, it is not fully understood what causes this complex neurodevelopmental disorder (1-3). A recent study by Filiano et al. (4) unraveled a novel mechanism regulating sociability, which may explain why some children develop ASD. The authors can convincingly demonstrate that an intact adaptive immune system is needed for correct social behavior.
The role of the immune system in brain pathology has been the focus of many studies in the past. Immune cells are crucially involved in regulating progression and outcome of brain disorders, such as infections, traumatic brain injury, stroke, multiple sclerosis, neurodegenerative diseases and others, while cells of the innate and adaptive immune system can have beneficial and detrimental effects in the brain (5,6). The importance of the adaptive immune system in regulating brain functions of healthy people has been recognized only recently. Under healthy conditions, circulating blood immune cells cannot infiltrate the CNS parenchyma due to an intact blood brain barrier. However, different leukocytes, including macrophages, T lymphocytes and B lymphocytes, are located in meningeal spaces, where they have been found to release substances capable of affecting brain functions. For example, the anti-inflammatory cytokine interleukin-4 released from meningeal T lymphocytes is involved in the regulation of learning and memory processes (7).
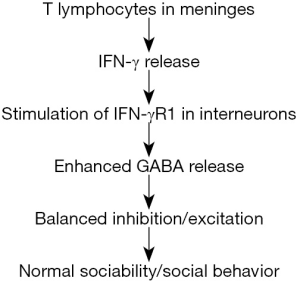
Filiano and colleagues (4) addressed the role of T lymphocytes in social behavior, with particular interest in sociability. In human, sociability is defined as the need/tendency to seek out companions and social relationships. To assess sociability in mice, Filiano et al. (4) determined the social preference of a mouse for another mouse over an object (8). Intriguingly, SCID mice, a mouse strain deficient in adaptive immune responses due to the absence of T lymphocytes, lacked social preference. These social deficits could be reversed by repopulation of SCID mice with T lymphocytes. To identify potential regulators of social behavior, Filiano et al. (4) searched for substances, which are released from T lymphocytes and are capable of crossing the blood-brain barrier. A gene set enrichment analysis (GSEA) was used to analyze transcriptomes of animals exposed to social stimuli, which resulted in the identification of upregulated interferon-γ (IFN-γ) regulated genes. To test the hypothesis that IFN-γ deficiency explains social deficits of SCID mice, behavior of IFN-γ-deficient mice was investigated. As found for SCID mice, IFN-γ-deficient mice lacked social preference for another mouse, while injection of recombinant IFN-γ restored social preference. Similarly, IFN-γ receptor 1 (IFN-γR1) knockout mice exhibited behavioral deficits, providing further evidence for the role of IFN-γ in mediating correct social behavior.
What is the mechanism by which IFN-γ released from meningeal T lymphocytes affects social behavior? It is well known that in brain pathology, IFN-γ causes microglial activation (9,10), while activated microglial cells release a variety of substances, such as pro- and anti-inflammatory cytokines, chemokines, reactive oxygen and nitrogen species, which can affect neuronal signaling and brain development (11,12). Furthermore, in the healthy brain, neuronal activity is constantly controlled by surveying microglia, while neuronal hyperactivity is reduced following prolonged contacts of microglial processes with axons or synapses (13). Thus, one could assume that IFN-γ activates microglial cells, which would then release substances capable of shaping social behavior via modulation of neuronal network activity. However, unexpectedly Filiano and colleagues (4) found that knockdown of IFN-γ receptors in microglia did not cause any changes in social behavior of mice when compared with that of wild-type mice. Further analyses revealed that IFN-γ receptors are not only expressed in microglia, but also in neurons. Knocking down neuronal IFN-γ receptors caused social deficits in mice similar to those observed in SCID mice and in IFN-γ knockout mice. Filiano et al. (4) found that IFN-γ receptors are expressed predominantly in GABAergic inhibitory neurons (interneurons) of prefrontal cortex layer I. In additional patch clamp studies the authors could demonstrate that application of IFN-γ enhanced GABAergic currents of these interneurons. Together, these observations led the authors to conclude that IFN-γ regulates neuronal circuits involved in social behavior by enhancing regional inhibition (4).
Disturbed neuronal circuit homeostasis due to an imbalance of excitation and inhibition is a hallmark of ASD, which is thought to be the reason for the lack of social preference in people with ASD (14). In addition, early-life epileptic seizures have been linked with deficits in social behavior and the development of ASD (15), which further suggests insufficient control of neuronal activity by GABAergic inhibitory neurons in individuals with ASD. Intriguingly, pathological features of brains from patients with ASD, namely hyper-connectivity in fronto-cortical/insular regions and hyper-responsiveness in prefrontal cortex were also detected by Filiano and colleagues (4) in brains of SCID mice and of IFN-γ-deficient mice. Furthermore, as described for some individuals with ASD, hyper-excitability of cortical circuits was detected in IFN-γR1-/- mice. Thus, findings of the study by Filiano et al. (4) performed on mice can be related to human ASD and may lead to the development of new therapeutic strategies for ASD.
In addition, the study by Filiano et al. (4) may have broader relevance for a better understanding of compensatory mechanisms occurring in the brain at early stages of neurological and/or psychiatric diseases. Due to increased blood brain barrier permeability, infiltrating T lymphocytes and enhanced IFN-γ concentrations have been found in the aged brain and at early stages of neurodegenerative diseases (16). While it has been suggested that infiltrating IFN-γ-producing T lymphocytes are exclusively responsible for promoting neuroinflammatory processes resulting in the progression of neurodegenerative diseases, these cells may also have a neuroprotective role. Release of small amounts of IFN-γ insufficient to trigger neuroinflammation, but sufficient to stimulate the activity of inhibitory GABAergic neurons could counteract neuronal hyperactivity, which is an early event observed in Alzheimer’s disease and other neurodegenerative diseases (17). Further studies are required to elucidate the precise role of T lymphocytes at early stages of brain pathology.
Some questions remain unanswered in the study by Filiano and colleagues (4). What is the mechanism causing accumulation of activated T lymphocytes in the meninges and which stimuli trigger IFN-γ release from T lymphocytes? Is IFN-γ constitutively required to regulate neuronal circuits/networks and social behavior, or are there critical moments in brain development which require IFN-γ release from meningeal T lymphocytes? Furthermore, it remains unclear why IFN-γ released from T lymphocytes does not activate microglial cells. One possible explanation is that the anti-inflammatory cytokine IL-4, which is also released from meningeal T lymphocytes (7), prevents microglial activation and subsequent neuroinflammation.
In summary, Filiano and colleagues (4) propose the following model to explain mechanisms by which the adaptive immune system regulates social behavior (Figure 1): meningeal CD4+ T lymphocytes release IFN-γ. After entering the brain parenchyma, IFN-γ binds to IFN-γ receptors of inhibitory neurons in layer I of the prefrontal cortex. IFN-γ receptor stimulation activates the STAT1 pathway, which leads to enhanced GABAergic neuronal activity. The activity of inhibitory neurons is required to ensure balanced excitation/inhibition in the prefrontal cortex, which will result in correct social behavior. Insufficient IFN-γ levels in the brain would reduce GABAergic inhibition leading to hyper-excitability of cortical networks and subsequent deficits in sociability. Together, this exciting new finding of the role of IFN-γ in regulating social behavior adds to our knowledge about interactions between the immune system and the central nervous system, which are crucial for developing and maintaining a healthy brain.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Public Health and Emergency. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.03.12). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barak B, Feng G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat Neurosci 2016;19:647-55. [Crossref] [PubMed]
- Masi A, DeMayo MM, Glozier N, et al. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci Bull 2017;33:183-93. [Crossref] [PubMed]
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ 2014;63:1-21.
- Filiano AJ, Xu Y, Tustison NJ, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 2016;535:425-9. [Crossref] [PubMed]
- Filiano AJ, Gadani SP, Kipnis J. Interactions of innate and adaptive immunity in brain development and function. Brain Res 2015;1617:18-27. [Crossref] [PubMed]
- Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol 2017;18:132-41. [Crossref] [PubMed]
- Derecki NC, Cardani AN, Yang CH, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 2010;207:1067-80. [Crossref] [PubMed]
- Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 2004;3:287-302. [Crossref] [PubMed]
- John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist 2003;9:10-22. [Crossref] [PubMed]
- Spencer NG, Schilling T, Miralles F, et al. Mechanisms Underlying Interferon-γ-Induced Priming of Microglial Reactive Oxygen Species Production. PLoS One 2016;11:e0162497 [Crossref] [PubMed]
- Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast 2013;2013:429815
- Bilimoria PM, Stevens B. Microglia function during brain development: New insights from animal models. Brain Res 2015;1617:7-17. [Crossref] [PubMed]
- Kato G, Inada H, Wake H, et al. Microglial Contact Prevents Excess Depolarization and Rescues Neurons from Excitotoxicity. eNeuro 2016;3(3
- Nelson SB, Valakh V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 2015;87:684-98. [Crossref] [PubMed]
- Lugo JN, Swann JW, Anderson AE. Early-life seizures result in deficits in social behavior and learning. Exp Neurol 2014;256:74-80. [Crossref] [PubMed]
- Korn T, Kallies A. T cell responses in the central nervous system. Nat Rev Immunol 2017;17:179-94. [Crossref] [PubMed]
- Stargardt A, Swaab DF, Bossers K. Storm before the quiet: neuronal hyperactivity and Aβ in the presymptomatic stages of Alzheimer's disease. Neurobiol Aging 2015;36:1-11. [Crossref] [PubMed]
Cite this article as: Eder C. Social behavior is regulated by the adaptive immune system. J Public Health Emerg 2017;1:41.