
A combined outbreak of Legionnaires’ disease and Pontiac fever related to an ultrasonic humidifier contaminated with Legionella pneumophila serogroup 1, Nanjing City, China, 2012
Introduction
Legionellosis is caused by Legionella bacteria and can result in a severe pneumonia disease [Legionnaires’ disease (LD)] and a mild febrile illness [Pontiac fever (PF)] (1). Since the first LD case was recognised in 1976 (2), most of reported outbreaks caused by Legionella have been determined as LD (3-8), and some of outbreaks have been diagnosed as PF (9-13), but rarely LD and PF occurred in an outbreak simultaneously (14-19). Legionellosis has been listed as a nationally notifiable disease in some of developing countries, such as European and USA, but not in China at present. And very few outbreaks of Legionellosis were reported from China (20) since the first LD case was confirmed in Nanjing City, 1982 (21).
The source of infection and transmission of Legionellosis outbreak was usually identified as inhalation of Legionella bacterial aerosols emitted from many different natural and artificial aquatic environments, such as cooling towers; water systems in hotels, homes, ships and factories; respiratory therapy equipment; fountains; misting devices and spa pools (1). Humidifiers, widely used misting devices in public places have been associated with some outbreaks of Legionellosis in health care facilities (22,23), homes (24-27), and factories (28).
On June 14, 2012, a physician from a comprehensive hospital in Nanjing City reported that more than 10 female workers outpatients from the same factory L became illness with fever, chest tightness, myalgia, non-productive cough, and headache within one day to the local Centre for Disease Control and Prevention (CDC). To verify and control the outbreak, identify the pathogen, and determine the source of infection and transmission mode, we conducted this epidemiological investigation.
Methods
Overview of factory L
Factory L, assembled the parts of washing machine, had a total of 45 staffs, including 5 managers working in office room, 19 outside stevedores, and 21 female workers in a closed workshop.
One ultrasonic humidifier and two cabinet air conditioners were set up for keeping the air conditions of temperature 20–25 °C and humidity 50–70% in the closed workshop in November 2010 (Figure 1).
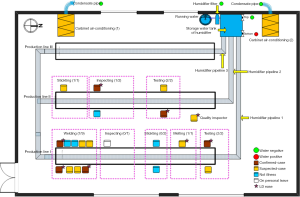
Five working days with 8 h per day from Monday to Friday every week were implementing in factory L. Food and drink was provided to the workers by the factory L in the working days.
Case definition
A suspected-case was defined as the staff from factory L had at least one abnormal result of five indicators in routine blood test (RBT) plus two or more of the following symptoms: fever, headache, fatigue, myalgia, non-productive cough and chest-tightness, during June 1st and 17th, 2012. RBT included five indicators testing of white blood cells (WBC), neutrophils (NE), lymphocytes (LY), monocytes (MO) and C-reactive protein (CRP). A confirmed-case was defined as a suspected-case with urine sample testing positive for Legionella pneumophila serogroup 1 (LP1) antigen. It was recognized for LD case as a suspected-case and a confirmed-case with the physician-diagnosed pneumonia (clinical features showed positive at least in both of two examinations: marked increases in chest X-radiography and moist crackles in lung auscultation), else for PF case.
Case finding and information collecting
A face to face interview was conducted to the staff in factory L by a structured questionnaire (29) on June 15, 2012. The information of demographic, clinical manifestations, and epidemiological characters were collected. The possible exposure risk for each staff was considered and asked about the attendance in factory L, travel history, hospital or public place visiting, respiratory disease complained and other respiratory illness patient contacting history, and water and food assumptions within 14 days before the onset of symptoms. Furthermore, the medical profiles of inpatients and outpatients were reviewed and the following information of the clinical symptoms and signs, results of RBT, chest X-radiography and lung auscultation, disease diagnosed, treatments and recovery were collected.
Environmental and settings investigation
The technology and chemical materials used in the closed workshop were checked by interviewing the manager and reviewing the production records, and no change was found within recent 6 months.
The two air conditioners were frequently used after installation, especially in winter (December, January and February) and summer (June, July and August).
However, the humidifier was only used three times from the operational and maintenance records of the humidifier: First for one hour operating in July 2011, second for half hour operating in January 2012, and third for three and half hour operating starting at 1:30 PM on July 13 2012. In order to estimate the potential relationship of attack rate (AR) and humidifier operating time, a retrospective study by using the same suspected-case definition but except for the non-available RBT results was conducted to find the cases from the 21 workers in the workshop during the 14 days after operating onset of humidifier in the first and second time.
This humidifier was connected with running water and released 18kg mist with a diameter of less than 10µm per hour by an auto-control and ultrasonic system (Figure 1). Three mist pipelines with the diameter of 110 mm were branched from the humidifier and were accompanied each production line in the closed workshop. The humidifier was filled up with running water since it first operating in July 2011 and had not been disinfected until this event occurred.
Hypothesis generating
After the cases searching and identification, all cases came from the closed workshop, no cases for managers and outside stevedores. Moreover, one worker from the closed workshop was not became illness as for one day on personal leave on June 13, 2012. And, no cases had the history of respiratory illness, respiratory illness patient contacting, travel, hospitals visiting, and public places visiting within 14 days before illness onset. Food and drinking water were provided to the workers by the factory L as the same as before. Therefore, the most possibility was the risk of the event sourced from the closed workshop.
Considering the cases showed the same clinical characters of the acute illness and infectious disease in this event, there was little possibility that this event was caused by the chemical materials due to a long-term occupational exposure.
Obviously, the settings of air conditioner and humidifier in the closed workshop attracted our attention and triggered the next clue of the event. From the field investigation, the two air conditioners were frequently used after installation, but the humidifier was only used three times, and especially for this time more than 10 female worker cases with an emergency appeared after humidifier operating several hours. An overview on the previous publications on the risk of the acute respiratory effects related to the humidifier indicated that the humidifier maybe as the most source of the infection, which leading the next work.
Samples colleting and testing
Urine samples were collected from all cases and tested for LP1 antigen (BinaxNOW®Legionella Urinary Antigen Card, LOT055074; Alere Inc., Waltham, MA, USA) on June 16, 2012. Sera samples from five inpatients were collected and tested for Legionella antigen using enzyme-linked immunosorbent assay and for Legionella antibody using gold immunochromatography assay on June 15, 2012.
Five water samples were collected to test Legionella bacteria on June 14, 2012. Among of them, two cooling water samples from each cabinet air conditioners separately, and the other three water samples were from the humidifier, one from the top of storage water tank and one from the bottom of storage water tank, and the last one from the humidifier filter (Figure 1). The water samples were concentrated by membrane filtration (0.2 µm), and filtered residues were resuspended in 1 mL sterile water. Of this suspension, 100-µL samples were cultured without dilution and after 10- and 100-fold dilutions on buffered charcoal yeast extract agar with alpha-ketoglutarate (BCYE-alpha) and a selective supplement with dyes and with and without the antibiotics polymyxin B, anisomycin, and vancomycin (Legionella MWY Selective Supplement SR 110, 111, and 118, Oxoid Ltd., Hampshire, England). Plates were incubated at 35 °C with increased humidity and examined regularly for the presence of colonies resembling Legionella species. In case of bacterial overgrowth, cultures were repeated after pretreatment by heating 30 min at 50 °C. Cultures were examined microscopically daily for 14 days. In case of persistent overgrowth, ceftazidime was added to the media. Colonies suspected of being Legionella were subcultured to BCYE-alpha agar. Identification was confirmed by biochemical tests (oxidase, catalase, and β-lactamase). We performed serotyping by using latex agglutination for L. pneumophila serogroups 1 and 2–14 (Legionella Latex Test, Oxoid Ltd.) and specific antibodies for serogroups 2 to 6 (Legionella antisera “Seiken”, Denka Seiken Co. Ltd., Tokyo, Japan).
Statically analysis and software
AR was calculated for overall and for different production lines, individually. Chi-square for a linear trend was used to test the dose-response of the risk of AR and the humidifier operating time.
All the Statistical analyses were performed using Epi Info™ 7.1.3.10 (Centers for Disease Control and Prevention, 2014/11/3) and Excel 2003. P value less 0.05 was regarded as significantly. The figures painting used the Microsoft Office Visio 2003.
Results
Epidemiological characters
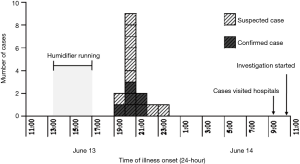
A total of 15 cases from the closed workshop, 9 suspected-cases (6 from production I and 3 from production II) and 6 confirmed-cases (4 from production I and 2 from production II), 8 LD cases (5 from production I, 2 from production II, and 1 was for the workshop quality inspector) and 7 PF cases (5 from production I and 2 from production II), with an overall AR of 75% (15/20, 1 worker from production line 1 was for personal leave on June 13, 2012) were identified for illness onset on June 13, 2012 (Figure 1). There was no significant difference of AR between the production line I (71%, 10/14) and production line II (80%, 4/5) (Chi Square =0.886, P>0.05). The median age of the 15 cases was 37 (range 24–43) years old, and the average working age was 16 (range 11–24) months (Table 1). There was no case observed from managers and outside stevedores.
Table 1
| ID | Age (years) | Working months | Suspected-case or confirmed-case | LD or PF$ | Outpatient or inpatient | Diagnosed as lung infection | Chest X-radiographyΨ | Lung auscultationγ | Serologic testingμ | Urine sample testingε |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | 12 | Confirmed | LD | Inpatient | Yes | Marked increases | Moist crackles | (−) | (+) |
| 2 | 34 | 12 | Confirmed | LD | Inpatient | Yes | Marked increases | Moist crackles | (−) | (+) |
| 3 | 39 | 18 | Confirmed | LD | Inpatient | Yes | Marked increases | Moist crackles | (−) | (+) |
| 4 | 42 | 12 | Confirmed | LD | Inpatient | Yes | Marked increases | Moist crackles | Antibody (+) | (+) |
| 5 | 24 | 12 | Confirmed | LD | Inpatient | Yes | Marked increases | Moist crackles | Antigen (+) | (+) |
| 6 | 38 | 19 | Confirmed | LD | Outpatient | Yes | Marked increases | Moist crackles | NA | (+) |
| 7 | 32 | 15 | Suspected | LD | Outpatient | Yes | Marked increases | Moist crackles | NA | (−) |
| 8 | 36 | 24 | Suspected | LD | Outpatient | Yes | Marked increases | Moist crackles | NA | (−) |
| 9 | 33 | 11 | Suspected | PF | Outpatient | No | Clear | Clear | NA | (−) |
| 10 | 37 | 14 | Suspected | PF | Outpatient | No | Clear | Clear | NA | (−) |
| 11 | 29 | 24 | Suspected | PF | Outpatient | No | NA | Clear | NA | (−) |
| 12 | 35 | 18 | Suspected | PF | Outpatient | No | NA | Clear | NA | (−) |
| 13 | 39 | 12 | Suspected | PF | Outpatient | No | NA | Clear | NA | (−) |
| 14 | 40 | 24 | Suspected | PF | Outpatient | No | Clear | Clear | NA | (−) |
| 15 | 40 | 16 | Suspected | PF | Outpatient | No | Clear | Clear | NA | (−) |
*, all cases became illness on June 13 2012; $, LD denoted for Legionnaires’ disease; PF denoted for Pontiac fever; Ψ, chest X-radiography were tested in 12 cases on June 14 2012; γ, lung auscultation was tested in all cases on June 14 2012; μ, serologic testing for
The illness onset of the index case was occurred in 7:00 PM on June 13, 2012, while the most of cases developed symptoms at 8:00 PM, the epi-curve showed a pattern of point source infection (Figure 2).
Clinical characters
Among the 15 cases, 5 were inpatients and 10 for outpatients, and 8 cases were diagnosed as lung infection by the physician (Table 1). Eight (53%) cases showed marked increases in both lungs in chest X-radiography and moist crackles in lung auscultation. None of the cases reported a previous respiratory illness within 14 days before illness onset. A total of 93% (14/15) had chest-tightness, and more than half had headache (80%), fatigue (80%), non-productive cough (80%), fever (73%), myalgia (67%), palpitation (60%), chills (53%) and nausea (53%) (Table S1). Symptoms of dizziness, dyspnoea, thoracalgia, aching limbs and vomiting were also found in some of the cases. Eight (53%) cases had an increasing frequency of urination with an average interval duration of 1.8 (range 1–3) h after illness onset to 6:00 AM in the next morning. Four of 5 indicators from the RBT results of all cases showed the 100% exceeded the range of reference value, except the indicator of MO (Table S2). All cases recovered after a 7-day antibiotic treatment of ceftriaxone, azithromycin and levofloxacin. Vitamins B and C, and Kalium chloratum (KCl) as the supplementary treatment were also given to the cases.
Laboratory results
Six (5 inpatients and 1 outpatients) of 15 cases urine samples collected at 3 days after illness onset were tested positive for LP1. Two cases of 5 inpatients were serologic testing positive for Legionella at 2 days after illness onset; one was for antibody positive and another for antigen positive (Table 1). Legionella bacteria was isolated from one of five water samples, collected from the bottom of storage water tank of the humidifier and confirmed as LP1 (Figure 1).
Risk estimated
A total of three times of the humidifier operating were recorded. The AR related to the third time (75%, 15/20) was higher than that in the previous two times (43%, 9/21) (Chi Square =4.45, P=0.04). A linear trend of the case numbers and the humidifier operating hours was observed among the three times (Chi Square =4.95, P=0.03).
Discussion
A combined outbreak of LD and PF related to an ultrasonic humidifier was detected in June 2012 in Nanjing City, China, which causing a total 15 cases of 8 LD cases and 7 PF cases. Environmental and epidemiological investigation suggested that the humidifier contaminated by the LP1 was probably the source of infection. As to our knowledge, it was the first report of a combined outbreak of LD and PF detected in China.
There were rarely reports demonstrated on the combined outbreak of LD and PF, especially in the workplace. Only several travel-associated (14,16-19) and community-acquired (15) combined outbreak of LD and PF were identified. We first described a combined outbreak of LD and PF occurred in an occupational place, which indicated the more concern need to be given in the further in the occupational place.
Humidifiers in the industries and factories are keeping for an appropriate humidity for meeting the production requirements. The occupational health risk of infection with L. pneumophila could increase when the humidifiers were not regularly cleaned and disinfected (28). We did not find any reference or guidelines on the humidifiers disinfection and cleaning in China through the internet searching. To prevent and control potential LD and others associated with humidifiers, regular cleaning and disinfecting guidelines of the humidifying equipment and devices to factories is needed. Moreover, the humidifier producers should provide relevant instructions for using humidifiers safely and healthily to purchasers.
There were some limitations to our study. Respiratory tract specimens of patients could not be collected for Legionella testing. We did not collect samples from humidifier pipelines for Legionella isolation and subtyping. Because we did not collect droplet samples from the humidifier for Legionella isolation, and we cannot simulate the exposure model of patients and cannot assess the risk of exposure and contamination. Among five hospitalised patients, only two were seropositive for Legionella after 2 days of illness onset, and among the urine samples collected from 15 patients 3 days after illness onset, only 40% were antigenic positive for LP1. A possible reason was the short duration (only 2–3 days) between exposure and sample collection. However, results consistent with LP1 were found both in the water sample from the humidifier and urine samples from six case-patients, which supports the evidence that this combined outbreak was most likely caused by the humidifier contaminated by LP1.
Conclusions
We reported the first combined outbreak of LD and PF in a closed workshop using an ultrasonic humidifier contaminated with LP1 in China. Regular cleaning and disinfection of humidifiers can be helpful to control and prevent Legionellosis outbreaks in an occupational environment. Model research is needed to provide more evidences of the transmission mode of humidifiers contaminated by L. pneumophila.
Table S1
| ID | Fever | Headache | Fatigue | Myalgia | Non-productive cough | Chest tightness | Palpitations | Chills | Nausea | Dizziness | Dyspnoea | Thoracalgia | Aching limbs | Vomiting | Frequent micturition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes (38.0 °C) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | No |
| 2 | Yes (38.0 °C) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| 3 | Yes (37.5 °C) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| 4 | Yes (38.0 °C) | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | No | Yes |
| 5 | Yes (37.7 °C) | Yes | No | No | Yes | Yes | No | No | No | No | No | Yes | No | No | Yes |
| 6 | Yes (39.8 °C) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | No | No | Yes |
| 7 | Yes (Self-reported) | Yes | Yes | No | Yes | Yes | No | No | No | Yes | No | No | No | No | Yes |
| 8 | Yes (Self-reported) | No | Yes | No | Yes | Yes | No | Yes | No | No | No | Yes | No | No | Yes |
| 9 | Yes (37.6 °C) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No |
| 10 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | No | Yes |
| 11 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No | No |
| 12 | No | Yes | No | No | No | Yes | Yes | No | No | No | Yes | No | No | Yes | No |
| 13 | Yes (Self-reported) | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | No | No | No | No | Yes |
| 14 | No | No | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | No | No |
| 15 | Yes (Self-reported) | No | Yes | No | Yes | Yes | No | Yes | No | Yes | No | No | No | No | No |
Table S2
| ID | CRP (mg/L) | WBC (×109/L) | MO (%) | NE (%) | LY (%) |
|---|---|---|---|---|---|
| 1 | 16.98 | 24.89 | 1.40 | 91.70 | 6.10 |
| 2 | NA | 24.65 | 1.40 | 92.30 | 5.60 |
| 3 | 32.40 | 23.80 | 1.70 | 90.50 | 6.70 |
| 4 | 29.10 | 22.18 | 1.70 | 88.80 | 8.20 |
| 5 | 38.00 | 21.46 | 1.80 | 85.20 | 11.70 |
| 6 | 26.40 | 18.90 | 2.40 | 87.00 | 9.10 |
| 7 | 39.30 | 20.11 | 2.10 | 77.40 | 19.90 |
| 8 | 18.60 | 13.00 | 2.00 | 84.10 | 12.70 |
| 9 | 41.10 | 20.14 | 1.60 | 89.30 | 8.60 |
| 10 | 22.64 | 20.45 | 2.60 | 84.70 | 11.00 |
| 11 | 34.90 | 19.15 | 2.30 | 88.20 | 8.20 |
| 12 | NA | 15.52 | 2.00 | 83.50 | 11.70 |
| 13 | 34.93 | 19.10 | 2.30 | 82.60 | 15.10 |
| 14 | 37.53 | 16.00 | 3.50 | 84.10 | 12.40 |
| 15 | 16.64 | 14.30 | 4.00 | 86.30 | 9.70 |
*, routine blood tests in all cases were tested on June 14 2012. The items included C-reactive protein (CRP), white blood cell (WBC), monocytes (MO), neutrophils (NE) and lymphocytes (LY). Reference value: CRP, 0–8.2mg/L; WBC, 4–10×109/L; MO, 3–12%; NE, 50–70%; LY, 20–40%. NA, not available.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant 81402749 and 81172626), Jiangsu Medical Innovation and Leading Team Grant (LJ201129), and Jiangsu Province Health Department Medical Research Face project (H201331). This study was partly supported by Natural Science Foundation of Jiangsu Province (BK20131014).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.09.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was considered to be part of a continuing public health event investigation by the Ministry of Health of Jiangsu Province, China and exempt from institutional review board assessment. In addition, we did not collect the personal or private information, including names and home address. All specimens were made anonymous before testing.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bartram J, Chartier Y, Lee JV, et al. editors. Legionella and the Prevention of Legionellosis. Geneva: World Health Organization (WHO), 2007.
- Fraser DW, Tsai TR, Orenstein W, et al. Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med 1977;297:1189-97. [Crossref] [PubMed]
- von Baum H, Härter G, Essig A, et al. Preliminary report: outbreak of Legionnaires’ disease in the cities of Ulm and Neu-Ulm in Germany, December 2009-January 2010. Euro Surveill 2010;15:19472. [PubMed]
- Haupt TE, Heffernan RT, Kazmierczak JJ, et al. An outbreak of legionnaires disease associated with a decorative water wall fountain in a hospital. Infect Control Hosp Epidemiol 2012;33:185-91. [Crossref] [PubMed]
- Coetzee N, Duggal H, Hawker J, et al. An outbreak of Legionnaires’ disease associated with a display spa pool in retail premises, Stoke-on-Trent, United Kingdom, July 2012. Euro Surveill 2012;17: [PubMed]
- Ulleryd P, Hugosson A, Allestam G, et al. Legionnaires’ disease from a cooling tower in a community outbreak in Lidköping, Sweden- epidemiological, environmental and microbiological investigation supported by meteorological modelling. BMC Infect Dis 2012;12:313. [Crossref] [PubMed]
- Vanaclocha H, Guiral S, Morera V, et al. Preliminary report: Outbreak of Legionnaires disease in a hotel in Calp, Spain, update on 22 February 2012. Euro Surveill 2012;17: [PubMed]
- Cheng VC, Wong SS, Chen JH, et al. An unprecedented outbreak investigation for nosocomial and community-acquired legionellosis in Hong Kong. Chin Med J (Engl) 2012;125:4283-90. [PubMed]
- Remen T, Mathieu L, Hautemaniere A, et al. Pontiac fever among retirement home nurses associated with airborne legionella. J Hosp Infect 2011;78:269-73. [Crossref] [PubMed]
- Cramp GJ, Harte D, Douglas NM, et al. An outbreak of Pontiac fever due to Legionella longbeachae serogroup 2 found in potting mix in a horticultural nursery in New Zealand. Epidemiol Infect 2010;138:15-20. [Crossref] [PubMed]
- Bauer M, Mathieu L, Deloge-Abarkan M, et al. Legionella bacteria in shower aerosols increase the risk of Pontiac fever among older people in retirement homes. J Epidemiol Community Health 2008;62:913-20. [Crossref] [PubMed]
- Modi A, Gardner J, Lighton L, et al. Pontiac fever outbreak associated with a spa-pool, United Kingdom, April 2008. Euro Surveill 2008;13: [PubMed]
- Burnsed LJ, Hicks LA, Smithee LM, et al. A large, travel-associated outbreak of legionellosis among hotel guests: utility of the urine antigen assay in confirming Pontiac fever. Clin Infect Dis 2007;44:222-8. [Crossref] [PubMed]
- Benin AL, Benson RF, Arnold KE, et al. An outbreak of travel-associated Legionnaires disease and Pontiac fever: the need for enhanced surveillance of travel-associated legionellosis in the United States. J Infect Dis 2002;185:237-43. [Crossref] [PubMed]
- Euser SM, Pelgrim M, den-Boer JW. Legionnaires' disease and Pontiac fever after using a private outdoor whirlpool spa. Scand J Infect Dis 2010;42:910-6. [Crossref] [PubMed]
- Thomas DL, Mundy LM, Tucker PC. Hot tub legionellosis: legionnaires’ disease and Pontiac fever after a point-source exposure to Legionella pneumophila. Arch Intern Med 1993;153:2597-9. [Crossref] [PubMed]
- Goldberg DJ, Fallon RJ, Green ST, et al. Pontiac fever in children. Pediatr Infect Dis J 1992;11:240-1. [Crossref] [PubMed]
- Girod JC, Reichman RC, Winn WC, et al. Pneumonic and nonpneumonic forms of legionellosis: the result of a common-source exposure to Legionella pneumophila. Arch Intern Med 1982;142:545-7. [Crossref] [PubMed]
- Goldberg DJ, Wrench JG, Collier PW, et al. Lochgoilhead fever: outbreak of non-pneumonic legionellosis due to Legionella micdadei. Lancet 1989;1:316-8. [Crossref] [PubMed]
- Shao ZJ. Surveillance and prevention of Legionnaires disease. Disease Surveillance 2005;20:281-2.
- Kang XM, Tang ZQ, Xia XR. One Legionnaires disease case report Med J Chin PLA 1982;7:240. (in Chinese).
- Hazard report. Mismatched electrical requirements between heated-wire breathing circuits and heated humidifiers can jeopardize patients. Health Devices 2009;38:267-9. [PubMed]
- Hashiguchi N, Hirakawa M, Tochihara Y, et al. Effects of setting up of humidifiers on thermal conditions and subjective responses of patients and staff in a hospital during winter. Appl Ergon 2008;39:158-65. [Crossref] [PubMed]
- Trenchs Sáinz De La Maza V, Domingo Garau A, García-Tornel Florensa S, et al. Domestic humidifiers. What do we know about them? An Esp Pediatr 2002;57:231-7. [PubMed]
- I've heard home humidifiers can cause health problems. Is this true? Mayo Clin Health Lett 2000;18:8. [PubMed]
- Kim EH, Ahn K, Cheong HK. Use of humidifiers with children suffering from atopic dermatitis. Environ Health Toxicol 2012;27:e2012004 [Crossref] [PubMed]
- Cheong HK, Ha M, Lee JH. Unrecognized bomb hidden in the babies' room: fatal pulmonary damage related with use of biocide in humidifiers. Environ Health Toxicol 2012;27:e2012001 [Crossref] [PubMed]
- Kateman E, Heederik D, Pal TM, et al. Relationship of airborne microorganisms with the lung function and leucocyte levels of workers with a history of humidifier fever. Scand J Work Environ Health 1990;16:428-33. [Crossref] [PubMed]
- Occupational Safety and Health Administration, U.S. Department of Labour, 2007. (Accessed on June 14, 2012). Available online: http://www.osha.gov/dts/osta/otm/otm_iii/otm_iii_7.html
Cite this article as: Zhou L, Cui L, Xiong L, Du X, Yu Y, Sheng X, Chen X. A combined outbreak of Legionnaires’ disease and Pontiac fever related to an ultrasonic humidifier contaminated with Legionella pneumophila serogroup 1, Nanjing City, China, 2012. J Public Health Emerg 2017;1:81.


