
Precision epidemiology of arboviral diseases
Introduction
Precision epidemiology is an emerging concept that brings to the forefront of research, the need for a new and more accurate/objective/efficient way of developing public health policies. Khoury et al. (1) defines it as “providing the right intervention to the right population at the right time”, seeking the use of new technologies for more accurate assessments of population health, disease risk and vulnerability, with the goal of improving the success of target preventive health programs. In this context, the terms precision and accuracy are used interchangeably, precision been defined as in the Oxford Dictionary: “the quality, condition, or fact of being exact and accurate”.
Arboviral diseases present big challenges and opportunities for the field of precision epidemiology. Challenges range from the development of more precise diagnostic methods at the individual level to more precise surveillance and interventions at the population level. Very importantly, it requires a more precise understanding of the dynamics of transmission in space and time. Epidemic processes are fundamentally different depending on the temporal and spatial scales at which they are observed and developing models that integrate local and global scales is an open field. The same occurs with the interventions, as they can take a long time to implement, like changing the sewage infrastructure of a city, or they can be focal, like application of aerial insecticides, or introducing sterile mosquitoes, or target vaccination. The former has a long lasting effect, while the latter have a focal effect. Building capacity to invest in both long term and focal interventions increases the resilience of communities and reduces disease burden. Optimizing the spatial and temporal application of these interventions is also part of the precision epidemiology. This process should be dynamic and adaptable according to epidemiological scenarios.
Among the arboviral diseases, dengue is an emblematic disease on which we can base this discussion. Endemic in more than 100 tropical and subtropical countries, it is a disease of international concern. This means that countries at risk should take routine and specific actions to build resilience against it (2). Dengue is caused by four antigenically distinct flaviviruses, transmitted by the mosquitoes of the genus Aedes (A. aegypti and A. albopictus). Dengue infection is commonly asymptomatic but when clinical manifestations occur, they will vary from mild to severe life-threatening symptoms. Severe dengue, in particular the dengue hemorrhagic fever (DHF), is an important cause of hospitalization and death in Asian and Latin American countries (3,4). A case-fatality rate of DHF less than 1% is expected in places with prompt diagnosis and treatment, but it can be as high as 20% in its absence (5,6). Predicting the progression of a case remains a challenge due to the non-specificity of the clinical presentation and the incomplete understanding of the underlying physiological mechanisms (7).
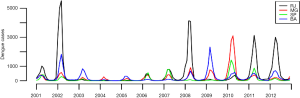
Predicting when and where the next dengue epidemic will strike is an important goal to direct control efforts. Despite its strong seasonal pattern, the magnitude and severity of dengue epidemics vary widely year-to-year and between provinces within a country. This pattern can be seen from Thailand (8) to Brazil (Figure 1). The explanations for this erratic behavior include the nonlinear effects of environmental factors (such as temperature and humidity), human factors (mobility and protective behavior) and immunological structure of the population with respect to the circulating strains. Spatial heterogeneities in the distribution of susceptible humans can also affect profoundly the likelihood of an epidemic depending on the local arrival of infective humans at the beginning of each season. Uncertainties regarding the next season impair our ability to prepare for and allocate resources to reduce disease burden.
A myriad of methods have been proposed to reduce the burden of dengue (Figure 2) from those aiming at reducing the risk of transmission and preventing new infections, to those aiming at preventing serious outcomes from infections that already occurred. The economic costs of these efforts are significant (9) and the effectiveness of the strategies is debatable (10).
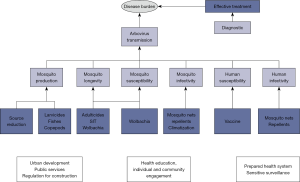
Due to the absence of an effective vaccine or specific treatment, historically, transmission control has been directed to the entomological component of the transmission cycle, via the application of methods for reducing mosquito vectorial capacity, abundance, longevity or susceptibility to the virus (Wolbachia infected mosquitoes) (11). Mechanical removal or chemical/biological treatment of breeding sites require household inspections by health agents. The effectiveness is low for many reasons: houses can be closed, breeding sites can be difficult to find, the tedious nature of the work may impact on the search effort. Even in perfect conditions, visiting every house of a large city can take too long and requires immense labor. The current perception is that strategies of mosquito population control by themselves are not enough, and integrated strategies are advocated as the only way to achieve success (12).
Increasing precision
Identifying key persons, houses and places
One underlying assumption of precision epidemiology is that a small subset of individuals, groups or places are responsible for the majority of disease burden and that directing control actions will enhance their impact in opposition to distributing them homogeneously in the population (13). In other words, this implies the assumption that individuals and places do not contribute equally to the total disease burden of a population, and by targeting a subset, we create positive externalities. We must keep in mind however, that precision in intervention does not imply precise outcomes and vice-versa. Sometimes focal and acute interventions may lead to global changes in the system and sometimes global action (such as wide vaccination coverage) is necessary to prevent a very specific event, such as disease invasion.
An important source of heterogeneity to be considered in precision epidemiology is the difference in individual's potential to transmit the disease to others. For many diseases, it has been observed that a few individuals are responsible for the majority of transmission, being referred to as super-spreaders (13). Well documented for directly transmitted diseases, superspreading is still a less explored concept in the context of vector-borne diseases. For these, superspreading events should be expected in places where there is a combination of a higher than average likelihood of a person infecting mosquitoes and vice versa.
Many physiological and environmental factors will contribute to increase disease spreading and theoreticians have scrutinized these parameters. Transmission will increase where the density of mosquitos per person is high, the incubation period in mosquitos is short, the longevity of mosquitos is long, the contact rate is effective and the viral transmission is high. Together, these parameters integrate the formula of the reproduction number, a well characterized measure of transmission in the mathematical literature (14).
Nguyet et al. (15) have shown that dengue patients who develop a higher and more lasting viremia, have a greater capacity to transmit the virus to mosquitos to which they are exposed to. Viremia lasts from 3.4 to 9.5 days and tend to be higher from one day before to two days after the onset of fever (16). Mosquitos that consume more viremic blood meal will also tend to end up with a more disseminated infection 12 days later, thus increasing the chance of transmitting the virus to a human (15). As a consequence, it is possible to conceive that a setting where individuals develop more viraemic infections has the potential to boost larger chains of transmission, considering everything else equal. Factors that can induce higher viremia include human factors such as lack of previous exposure, low immunity response, and virological factors such as strain of dengue virus (15,17).
Not only humans but also mosquitoes show heterogeneity in their response to viral infection. Mosquitos that were bred under stressful conditions such as in the presence of resource limitation or competitors will tend to develop more intense viral load (18). These environmental conditions will affect the vectorial capacity of Aedes mosquitoes, further interfering with the transmission chain. Mosquito biting is also not random, as human attractiveness may be related to sex, skin surface area, and behavior (19). Local variations in temperature can also contribute to enhance foci of transmission. At warm temperatures, the reproduction of Aedes mosquitoes is more efficient and leading to higher abundance. Also, the incubation period of the virus is reduced from 15 to 7 days, and the overall vectorial capacity tends to increase (20). All these contribute to reduce the chance of stochastic population extinction (when a small population randomly fluctuates towards zero) and to increase the speed of transmission.
Even if [human and mosquito] hosts were equally susceptible, superspreading events should occur where there is a high rate of contact between (human and mosquito) hosts. Spatial variation in mosquito abundance is well known, as mosquitoes tend to be clustered. For example, in Australia, 2−5% of the households contained more than 50% of the producing breeding sites (21) while 92% of pupae were found in 5% of houses in Colombian cities (22). As adults, mosquitos show low mobility, rarely flying more than 100 meters away from their breeding site during their lifetime. Together, all these facts suggest that a good target for precision epidemiology is identifying areas with a high propensity for transmission, that is, small areas characterized by high mosquito abundance, high human density (in particular in the presence of susceptible individuals capable of producing high viremia), where there are environmental conditions for the frequent contact between humans and mosquitoes and arrival of viruses.
Human movement patterns have being associated with superspreading events as well. Humans are more mobile than mosquitos and their movement patterns are considered important determinants for the introduction of viruses into susceptible populations. The importance of human movement range from global patterns leading to the expansion of the dengue endemic frontier (23), to very local level explaining differences in seroprevalence between a peripheric and more central area of a city (24).
There are some attempts in the literature at classifying areas according to their importance for dengue control. One method being proposed is the vulnerability mapping whose goal is to develop and map a composite indicator that summarises the complex web of factors and interactions that mediate exposure, susceptibility, and ability to recover from dengue (25). The point of start is interesting as it involves stating a web of factors, from proximal to distal causes, from land use to education, acknowledging the complexity of the problem. However, the summarization of such complexity into a single indicator to be mapped is still an important issue to be considered. Actually, we see a great opportunity for dialogue between this multidisciplinary and complex thinking approach and the long tradition in epidemiology for representing complex systems through mathematical and statistical models which can handle the nonlinearities and multiscale nature of the system.
Acting with precision
Monitoring vector populations is an activity that is routinely carried out in many countries at a high cost. Larval surveys produce indices that are used for stratifying areas according to the risk of outbreaks, the premise condition index above 1% being an indicative of risk (26). Despite the lack of evidence of effectiveness, most countries still rely on larval household surveys to produce Stegomyia indices, which include the premise index, Breteau index and container index. In Brazil, as in other areas, surveys are carried out 2–4 times a year, using a space unit of ca. 2,000 households.
Attempts to increase the precision of mosquito-positive container detection are not new. In 1995, Tun-Lin et al. (21) in Queensland, Australia, proposed a method for the identification of premises with higher chance of being positive, based on the finding that few premises harbor the majority of breeding sites. The Premise Condition Index (PCI) was constructed from easily observed features of a premise, such as its conservation status and the degree of shading. The method was applied to different settings with apparent success (22) but no study has been designed to evaluate the impact of such strategy in a controlled experiment. For example, it is not known what is the impact of removing a proportion of positive containers only, considering that these mosquitoes have very plastic behavior (27) and despite the tendency to show spatial clustering, mosquitos clusters can be very unstable (28).
Nowadays, the opportunities to improve the identification of high risk premises vary from using drones, to satellite images (29). These initiatives are still in the conceptual and pilot phases but are promising as tools for detecting areas with a higher chance of containing breeding sites.
Another approach to increase the precision of control efforts is to involve the community. Ideally, citizens are the ones that better know the problems where they live and important partners to think about solutions. In Cuba (30), community working groups were organized with the aid of volunteers, family medicine and vector control agents to intervene in public areas by eliminating breeding sites, transforming garbage areas into vegetable gardens, repairing broken water pipes and covering water containers, source reduction through periodic inspection of houses, and chemical treatment, health education and law enforcement of mosquito control practices. In four other countries of Latin America (31), cluster randomized trials have been developed to assess the impact of community engagement strategies involving health education, clean-up campaigns, workshops on reducing mosquito indices. These studies show that empowering the community can have an impact on mosquito infestation and precision epidemiology can contribute to take this strategy beyond the studies. New digital technologies are great for fostering participatory activities and these could be useful for increasing the efficacy of community engagement (32).
A number of technologies for monitoring the presence of pathogenic viruses in mosquitoes are also being evaluated. For example, quick tests for DENV NS1 antigen using carbon nanotube based nanobiosensors (33) can potentially lead to real-time tracking of DENV in mosquitoes captured in mosquito traps. Near-infrared spectroscopy has also shown great potential to non-invasively detect viral infections, being able to distinguish between DENV, ZIKV and CHIKV infections in Aedes aegypti mosquitoes (34), as well as the presence of Wolbachia (35) which is being introduced in several Aedes populations around the world in order to block the transmission of multiple viruses.
Optimizing the timing of interventions
We cannot underestimate the importance of reducing the delays and costs of reporting events of public health relevance. Incomplete and/or delayed information will undermine any efforts to deliver early warnings, a central topic of precision epidemiology. Hadler et al. (35) concluded after an assessment of the arboviral surveillance in the United States, that current surveillance methods are not capable of providing the rapid detection required for an effective response to arboviral threats.
Dengue surveillance in most countries relies on the passive reporting of cases from patients seeking care. Reporting delay can vary from place to place reflecting health care providers adherence to the reporting protocol, as well as the access of patients to health care. This reporting delay plagues many surveillance systems that rely on imperfect reporting systems. Recently, we developed a statistical method to estimate current disease incidence from data with notification delays (36). Such approach can increase the precision of alert models and it is used by the Infodengue early warning system implemented in Brazil (www.info.dengue.mat.br).
In the last years, non-traditional sources of data have been considered to improve the timeliness of early warning systems. For example, mention of dengue in certain social networks has being shown to correlate with dengue incidence and have predictive power (37). Online data sources have the advantage of being low-cost, almost instantaneous. The disadvantage is their sensibility to spatial and temporal changes in human social behavior. Used with care, they are valuable tools for early warning.
We believe that the challenge of integrating data of different kinds at a global scale will be the last roadblock to be removed before precise management of global health threats can be achieved. The way to tackle this challenge are through the local integration of data and adhering to open-standards storage formats and protocols for data exchange. Once data are organized and accessible on the local scale, integrating them for analyses on a global scale will become possible, though not automatic. There will be political barriers to the free flow of information that are hard to overcome, despite the clear benefits of an integrated system. A barrier to be surmounted is the bottleneck posed by the centralized systems that are responsible for notification data collection, aggregation and verification which impose huge delays on data acquisition. Issues of privacy and provenance of health data have always been responsible for serious limitation to the speed and reach (who can access) of health data. With the advent of distributed ledger technologies, also known as Blockchain, some of these issues can now be solved, leading to a much more efficient information workflow (38). By applying blockchain technologies, publicization of personal health records is no longer an issue since it can be reported in encrypted form. Provenance can be attested by cryptographic signatures, and the records can be made tamper proof thanks to the immutability of records published on the blockchain. Workflows, as those described by Coelho (38), show how descriptive statistics can be calculated on the records without the need to decrypt the data, which means that, as before, only authorized health professionals will have access to the full data, but anyone will be able to have access to the statistics of the full data, as soon as it is reported.
Conclusions
Pursuing greater precision in epidemiological monitoring and control remains a difficult but worthwhile challenge. This essay, not intended as a comprehensive review, describes some of the avenues that are open towards a precision dengue epidemiology and emphasizes the importance of being aware of the indirect effects from any intervention simply due to hidden interactions within such a complex system. There is no reason to believe that controlling health locally will be any easier than locally controlling other complex systems, such as the weather or ocean tides. This consideration should be taken as evidence that a lot more needs to be invested into knowledge, surveillance and diagnostic tools. The better we know, the better we will be able to control. Therefore, more investment is needed to improve the measuring aspect of precision epidemiology. Currently, monitoring disease burden is left as a government task mostly disconnected from research and innovation. In the age of information when we often hear that “data is the new oil”, it is urgent that we develop the concept of a public health data marketplace which will provide the much needed economic incentive to the development of better surveillance, data management and analysis tools which are key to advancing the precision epidemiology agenda. As an example of the power of markets, the pharmaceutical and health care markets provide effective economic incentives for investing in the research and development of new control measurements such as vaccines and new pharmacological agents. When it comes to surveillance and diagnostics, this market dynamic is still the missing engine of progress.
Acknowledgments
Funding: The authors would like to acknowledge financial support from the Brazilian research council CNPq (grant 305553/2014-3).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Antoine Flahault, Olga De Santis) for the series “Precision Infectious Disease Epidemiology” published in Journal of Public Health and Emergency. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2018.12.03). The series “Precision Infectious Disease Epidemiology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khoury MJ, Iademarco MF, Riley WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med 2016;50:398-401. [Crossref] [PubMed]
- Glonti K, Gordeev VS, Goryakin Y, et al. A Systematic Review on Health Resilience to Economic Crises. PLoS One 2015;10:e0123117 [Crossref] [PubMed]
- Clark DV, Mammen MP Jr, Nisalak A, et al. Economic impact of dengue fever/dengue hemorrhagic fever in Thailand at the family and population levels. Am J Trop Med Hyg 2005;72:786-91. [Crossref] [PubMed]
- Teixeira MG, Costa MC, Coelho G, et al. Recent shift in age pattern of dengue hemorrhagic fever, Brazil., Recent Shift in Age Pattern of Dengue Hemorrhagic Fever, Brazil. Emerg Infect Dis 2008;14:1663. [Crossref] [PubMed]
- Chang K, Huang CH, Lee IK, et al. Differences in Mortality and Clinical Manifestations of Dengue Hemorrhagic Fever in Taiwan in Different Years: A Comparison for Cases in 2014 and 2015 Epidemics. Am J Trop Med Hyg 2017;97:361-8. [Crossref] [PubMed]
- Wei HY, Shu PY, Hung MN. Characteristics and Risk Factors for Fatality in Patients with Dengue Hemorrhagic Fever, Taiwan, 2014. Am J Trop Med Hyg 2016;95:322-7. [Crossref] [PubMed]
- Nedjadi T, El-Kafrawy S, Sohrab SS, et al. Tackling dengue fever: current status and challenges. Virol J 2015;12:212. [Crossref] [PubMed]
- Campbell KM, Lin CD, Iamsirithaworn S, et al. The complex relationship between weather and dengue virus transmission in Thailand. Am J Trop Med Hyg 2013;89:1066-80. [Crossref] [PubMed]
- Gubler DJ. The economic burden of dengue. Am J Trop Med Hyg 2012;86:743-44. [Crossref] [PubMed]
- Bouzid M, Brainard J, Hooper L, et al. Public Health Interventions for Aedes Control in the Time of Zikavirus-A Meta-Review on Effectiveness of Vector Control Strategies. PLoS Negl Trop Dis 2016;10:e0005176 [Crossref] [PubMed]
- Caragata EP, Dutra HL, Moreira LA. Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol 2016;32:207-18. [Crossref] [PubMed]
- Luz PM, Vanni T, Medlock J, et al. Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet 2011;377:1673-80. [Crossref] [PubMed]
- Stein RA. Super-spreaders in infectious diseases. Int J Infect Dis 2011;15:e510-e513. [Crossref] [PubMed]
- Massad E, Coutinho FAB. Vectorial capacity, basic reproduction number, force of infection and all that: formal notation to complete and adjust their classical concepts and equations. Mem Inst Oswaldo Cruz 2012;107:564-7. [Crossref] [PubMed]
- Nguyet MN, Kien DTH, Tuan TV, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci 2013;110:9072-7. [Crossref] [PubMed]
- Chan M, Johansson MA. The incubation periods of dengue viruses. PloS One 2012;7:e50972 [Crossref] [PubMed]
- Tricou V, Minh NN, Farrar J, et al. Kinetics of Viremia and NS1 Antigenemia Are Shaped by Immune Status and Virus Serotype in Adults with Dengue. PLoS Negl Trop Dis 2011;5:e1309 [Crossref] [PubMed]
- Alto BW, Lounibos LP, Mores CN, et al. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc Biol Sci 2008;275:463-71. [Crossref] [PubMed]
- Liebman KA, Stoddard ST, Reiner RC Jr, et al. Determinants of heterogeneous blood feeding patterns by Aedes aegypti in Iquitos, Peru. PLoS Negl Trop Dis 2014;8:e2702 [Crossref] [PubMed]
- Codeço CT, Villela DAM, Coelho FC. Estimating the effective reproduction number of dengue considering temperature-dependent generation intervals. Epidemics 2018;25:101-11. [Crossref] [PubMed]
- Tun-Lin W, Kay BH, Barnes A. Understanding productivity, a key to Aedes aegypti surveillance. Am J Trop Med Hyg 1995;53:595-601. [Crossref] [PubMed]
- Nogueira LA, Gushi LT, Miranda JE, et al. Application of an alternative Aedes species (Diptera: Culicidae) surveillance method in Botucatu city, São Paulo, Brazil. Am J Trop Med Hyg 2005;73:309-11. [Crossref] [PubMed]
- Lana RM, da Costa Gomes MF, de Lima TFM, et al. The introduction of dengue follows transportation infrastructure changes in the state of Acre, Brazil: A network-based analysis. PLoS Negl Trop Dis 2017;11:e0006070 [Crossref] [PubMed]
- Honório NA, Nogueira RMR, Codeço CT, et al. Spatial evaluation and modeling of Dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis 2009;3:e545 [Crossref] [PubMed]
- Dickin SK, Schuster-Wallace CJ, Elliott SJ. Developing a vulnerability mapping methodology: applying the water-associated disease index to dengue in Malaysia. PLoS One 2013;8:e63584 [Crossref] [PubMed]
- Tun-Lin W, Kay BH, Barnes A. The Premise Condition Index: A Tool for Streamlining Surveys of Aedes aegypti. Am J Trop Med Hyg 1995;53:591-4. [Crossref] [PubMed]
- Russell BM, McBride WJH, Mullner H, et al. Epidemiological significance of subterranean Aedes aegypti (Diptera: Culicidae) breeding sites to dengue virus infection in Charters Towers, 1993. J Med Entomol 2002;39:143-5. [Crossref] [PubMed]
- LaCon G, Morrison AC, Astete H, et al. Shifting patterns of Aedes aegypti fine scale spatial clustering in Iquitos, Peru. PLoS Negl Trop Dis 2014;8:e3038 [Crossref] [PubMed]
- Bergquist R. New tools for epidemiology: a space odyssey. Mem Inst Oswaldo Cruz 2011;106:892-900. [Crossref] [PubMed]
- Toledo ME, Rodriguez A, Valdés L, et al. Evidence on impact of community-based environmental management on dengue transmission in Santiago de Cuba. Trop Med Int Health 2011;16:744-7. [Crossref] [PubMed]
- Quintero J, García-Betancourt T, Caprara A, et al. Taking innovative vector control interventions in urban Latin America to scale: lessons learnt from multi-country implementation research. Pathog Glob Health 2017;111:306-16. [Crossref] [PubMed]
- Viennet E, Ritchie SA, Williams CR, et al. Public health responses to and challenges for the control of dengue transmission in high-income countries: four case studies. PLoS Negl Trop Dis 2016;10:e0004943 [Crossref] [PubMed]
- Wasik D, Mulchandani A, Yates MV. Point-of-Use Nanobiosensor for Detection of Dengue Virus NS1 Antigen in Adult Aedes aegypti: A Potential Tool for Improved Dengue Surveillance. Anal Chem 2018;90:679-84. [Crossref] [PubMed]
- Fernandes JN, Dos Santos LMB, Chouin-Carneiro T, et al. Rapid, noninvasive detection of Zika virus in Aedes aegypti mosquitoes by near-infrared spectroscopy. Sci Adv 2018;4:eaat0496.
- Hadler JL, Patel D, Nasci RS, et al. Assessment of arbovirus surveillance 13 years after introduction of West Nile virus, United States. Emerg Infect Dis 2015;21:1159. [Crossref] [PubMed]
- Bastos L, Economou T, Gomes M, et al. Modelling reporting delays for disease surveillance data. ArXiv170909150 Stat [Internet] 26 de setembro de 2017 [citado 15 de agosto de 2018].
- Marques-Toledo CA, Degener CM, Vinhal L, et al. Dengue prediction by the web: tweets are a useful tool for estimating and forecasting Dengue at country and city level. PLoS Negl Trop Dis 2017;11:e0005729 [Crossref] [PubMed]
- Coelho FC. Optimizing Disease Surveillance by Reporting on the Blockchain. bioRxiv 2018;278473
Cite this article as: Coelho FC, Codeço CT. Precision epidemiology of arboviral diseases. J Public Health Emerg 2019;3:1.


