Diagnostic value of C-reactive protein, IgG, and IgA for detection of pulmonary tuberculosis: a case-control study from Eastern China
Introduction
Globally, tuberculosis is one of the top 10 causes of death and the leading cause from a single infectious agent. Successful diagnosis and treatment of pulmonary tuberculosis averts millions of deaths each year (an estimated 54 million over the period 2000–2017). In 2018, a total of 7.0 million new cases were reported, of which China accounted for 9% (1). However, there are still large and persistent gaps in detection and treatment of tuberculosis patients. Currently, >30% of tuberculosis patients are not diagnosed globally. Although underdiagnosis and underreporting both contribute to these “missing” cases, underdiagnosis is considered critical to improving tuberculosis control in endemic settings (1).
There is an urgent need for screening and testing strategies that provide point-of-care test results (2). Sputum smear microscopy and mycobacterial culture requires weeks, high quality sampling, and suitable laboratory facilities (1,3). Besides, the only rapid detection method for Mycobacterium tuberculosis (MTB) recommended by WHO has not been implemented in many areas of China, and one of the reasons is its high cost (4).
Recently, serodiagnostic assays have become more available for point-of-care translation (5). Detection of blood-based biomarkers remains an appealing approach. C-reactive protein (CRP), an acute phase protein synthesized by liver hepatocytes, is often proposed for pulmonary tuberculosis diagnosis (6,7). In response to infection or tissue inflammation, the immunity is mainly stimulated by interleukin 6, interleukin 1β and tumor necrosis factor α (8). An observational case-control study indicated that expectoration, chest pain, wasting, and culture count positively associated with CRP (9). Several reports suggested that serum antibodies in tuberculosis patients reacted strongly with M. tuberculosis membrane proteins (10). Some of them have reported that IgA and IgG levels were higher in pulmonary tuberculosis patients than in healthy people (11-13). A recent systematic review stated that CRP was a valid tool for screening persons living with HIV (14) however its use in tuberculosis patients without HIV is not clear.
To improve our understanding of the diagnostic value of CRP diagnostics, we performed a case-control study in Eastern China and investigated the ability of CRP, alone and in-combination with IgG and IgA between pulmonary tuberculosis patients and healthy population. We presented the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jphe-20-38).
Methods
Study population
Our study evaluated pulmonary tuberculosis patients accessing the Second Hospital of Nanjing between September 2018 and June 2019. Patients were enrolled if they had chest radiographical images compatible with pulmonary tuberculosis. After the X-ray, patients underwent a serious of bacteriological examinations for pulmonary tuberculosis diagnosis, including routine acid-fast bacilli (AFB) smear microscopy, mycobacterial culture, and Xpert MTB/RIF. Tuberculosis diagnosis was conducted by clinical specialists according to the National Diagnostic Criteria (Table S1).
The control group included healthy individuals visiting the Yixing People’s Hospital between October 2019 and November 2019 for a physical examination. The pulmonary tuberculosis group and control group were matched 1:1 by age and sex. Exclusion criteria for controls included a history of tuberculosis and other infections, a history of tumor, chronic diseases, immune system diseases, using of tuberculosis treatment drugs.
Immunoassays
Serum specimens of both pulmonary tuberculosis group and control group were collected by standard venipuncture in sodium heparin tubes. Concentrations of CRP, IgG and IgA were measured in duplicate serum samples using the turbidimetric inhibition immunoassay (ERKN, China) according to the manufacturer’s instructions.
CRP started with mixing up 2 µL serum specimen and 240 µL ammonium chloride buffer (0.2 mmol/L) together and incubated at 37 °C for 3–5 minutes. Secondly, 60 µL anti-human CRP latex particle were added and incubated at 37 °C for 10 seconds. Lastly, read absorbance A1 and A2 at 570 nm (550–590 nm) and 700 nm. For IgG testing, we blended 2 µL serum specimen with 250 µL polyethylene glycol (<4%) first and incubated at 37 °C for 5 minutes. Read absorbance A1 at 405 nm. Then 50 µL anti-human IgG was added and the samples was incubated at 37 °C for 5 minutes. Read absorbance A2 at 700 nm. The test of IgA was similar with IgG, replacing polyethylene glycol and anti-human IgG with PEG4 buffer and anti-human IgA, respectively. The A1 and A2 for IgA were 340 and 700 nm.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was considered part of routine public health surveillance and, due to this, was considered exempt from ethical approval. All data were de-identified prior to use, and identifying information was not available. Written informed consent was obtained from all eligible TB patients.
Statistical analysis
Data was entered with EpiData 3.1 (EpiData Association, Odense, Denmark) and analyzed using Stata 15.0 (Stata Corp., College Station, TX, USA) and MedCalc MedCalc (Version 19.1.7, MedCalc Software, Mariakerke, Belgium). Continuous variables were summarized as medians with interquartile range (IQR). Categorized variables were described using frequencies and proportions. We used Wilcoxon rank sum tests to compare the equality of the CRP, IgG and IgA values between groups. According to the Standards for Reporting Diagnostic Accuracy (STARD) guidelines (15), we estimated the values of sensitivity, specificity, positive and negative likelihood ratio of CRP for the diagnosis of pulmonary tuberculosis, bacteriologically positive and negative tuberculosis for different CRP cut-off points. Receiver Operating Characteristic (ROC) curves statistics were used to compare the diagnostic accuracy of CRP alone and with IgG and IgA.
Results
Demographic characteristics of all the participants
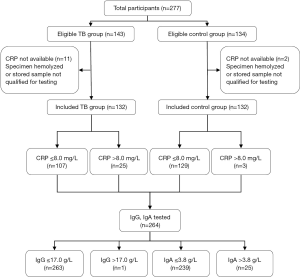
During September 2018 and November 2019, we consecutively enrolled 132 pulmonary tuberculosis cases in the Second Hospital of Nanjing and 132 healthy control who visited the Yixing People’s Hospital matched 1:1 for age and sex (Figure 1). We excluded those participants whose CRP values were not available. Of all the participants included, there were 80 (60.6%) males and 52 (39.4%) females. No significant statistics differences were found between pulmonary tuberculosis group and control group in race, gender, age, body mass index (BMI), occupation, smoking and drinking status (Table 1). There were 64 (48.5%) bacteriological negative pulmonary tuberculosis and 68 (51.5%) bacteriological positive pulmonary tuberculosis (Table S2).
Table 1
| Characteristics | TB group, n (%) | Control group, n (%) | χ2/Z | P value |
|---|---|---|---|---|
| Gender | 0.000 | 1.000 | ||
| Male | 80 (60.6) | 80 (60.6) | ||
| Female | 52 (39.4) | 52 (39.4) | ||
| Race | 0.680 | 0.409 | ||
| Han nationality | 128 (97.0) | 130 (98.5) | ||
| Others | 4 (3.0) | 2 (1.5) | ||
| Age, years | 0.028 | 0.982 | ||
| ≤25 | 34 (25.8) | 33 (25.0) | ||
| 26–39 | 38 (28.8) | 37 (28.0) | ||
| 40–55 | 29 (22.0) | 31 (23.5) | ||
| >55 | 31 (23.5) | 31 (23.5) | ||
| Body mass index, kg/m2 | 3.687 | 0.055 | ||
| <18.5 | 22 (16.7) | 18 (13.6) | ||
| 18.5 to <24 | 82 (62.1) | 69 (52.3) | ||
| 24 to <28 | 24 (18.2) | 42 (31.8) | ||
| ≥28 | 4 (3.0) | 3 (2.3) | ||
| Occupation | 1.043 | 0.307 | ||
| Agricultural labor | 10 (7.6) | 6 (4.5) | ||
| Nonagricultural labor | 122 (92.4) | 126 (95.5) | ||
| Smoking status | 3.272 | 0.071 | ||
| Never smoked | 93 (70.5) | 79 (59.8) | ||
| Ever smoked | 39 (29.5) | 53 (40.2) | ||
| Alcohol drinking | 2.967 | 0.085 | ||
| No | 106 (80.3) | 94 (71.2) | ||
| Yes | 26 (19.7) | 38 (28.8) | ||
| Median CRP (IQR), mg/L | 1.70 (0.73–4.38) | 0.90 (0.50–2.08) | −3.661 | <0.0001 |
| Median IgG (IQR), g/L | 9.70 (8.28–11.93) | 10.46 (9.62–12.25) | −2.740 | 0.006 |
| Median IgA (IQR), g/L | 2.19 (1.56–2.95) | 2.09 (1.59–2.68) | −0.550 | 0.582 |
PTB, pulmonary tuberculosis; IQR, interquartile range; BMI, body mass index; CRP, C-reactive protein.
Comparisons of CRP, IgG, and IgA values between groups
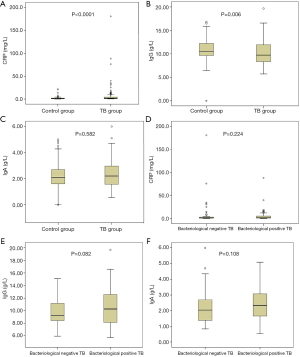
To explore the relationship between CRP, IgG and IgA and mycobacterial load, we used Wilcoxon rank sum tests to compare the equality of values between the bacteriological positive pulmonary tuberculosis and bacteriological negative pulmonary tuberculosis. As shown in Figure 2A,B,C refers to the comparison of CRP, IgG and IgA between control group and TB group, and Figure 2D,E,F refers to comparison of CRP, IgG and IgA between the bacteriological positive TB and bacteriological negative TB. There were no significant statistics differences of these two groups (P>0.05). Between pulmonary tuberculosis group and control group, we found that CRP values and IgG values had statistical differences (P<0.0001, P=0.006, respectively).
Utility of different CRP cut-off points for tuberculosis diagnosis
For CRP, we estimated the sensitivity, specificity, positive and negative likelihood ratio for ruling in or out a pulmonary tuberculosis diagnosis (Table 2). For bacteriologically negative patients, relatively higher specificity was available when CRP >5 mg/L, which was 95.31% and the sensitivity was quite low (15.65%). With the CRP cut-off points set as 5 mg/L, the values of +LR and −LR were 4.25 and 0.80 in bacteriological positive group. When CRP >8 mg/L, the sensitivity and specificity for pulmonary tuberculosis group and control group were 12.50% and 95.31%, respectively.
Table 2
| Group | Subcategory | CRP (mg/L) | |||
|---|---|---|---|---|---|
| >0.5 | >1 | >5 | >8 | ||
| All | Sensitivity | 84.85 (77.61–90.48) | 62.88 (54.06–71.18) | 15.63 (7.83–26.95) | 12.50 (5.66–23.24) |
| Specificity | 25.76 (18.50–34.13) | 54.55 (45.72–63.29) | 92.19 (82.70–97.49) | 95.31 (86.98–99.01) | |
| +LR | 1.14 | 1.38 | 2.00 | 2.67 | |
| −LR | 0.59 | 0.68 | 0.92 | 0.92 | |
| Bacteriological negative group | Sensitivity | 88.24 (78.09–94.75) | 56.25 (43.32–68.63) | 15.63 (7.81–26.94) | 12.50 (5.63–23.25) |
| Specificity | 32.35 (21.52–44.83) | 51.56 (38.73–64.24) | 95.31 (86.92–99.07) | 95.31 (86.92–99.01) | |
| +LR | 1.30 | 1. 16 | 3.33 | 2.67 | |
| −LR | 0.36 | 0.85 | 0.89 | 0.92 | |
| Bacteriological positive group | Sensitivity | 81.25 (69.54–89.91) | 69.12 (56.76–79.85) | 25.00 (15.31–37.07) | 23.53 (14.16–35.43) |
| Specificity | 18.75 (10.11–30.48) | 57.35 (44.84–69.34) | 94.12 (85.64–98.46) | 100.00 (94.78–100.00) | |
| +LR | 1.00 | 1.62 | 4.25 | – | |
| −LR | 1.00 | 0.54 | 0.80 | 0.76 | |
CRP, C-reactive protein; +LR, positive likelihood ratio; −LR, negative likelihood ratio.
Comparisons of diagnostic accuracy of CRP, IgG and IgA
To further explore the diagnostic value of CRP, we combined the added value of IgG and IgA with CRP. For all the participants included, the area under the curve (AUC) of CRP alone was 0.63 (95% CI, 0.59–0.69), combined CRP with IgG was 0.69 (95% CI, 0.63–0.74), combined CRP with IgG and IgA was 0.69 (95% CI, 0.63–0.74). There were no statistically significant differences between the three assays (P>0.05). As shown in Figure 3, the AUC of CRP alone was 0.57 (95% CI, 0.48–0.60), combined CRP with IgG was 0.73 (95% CI, 0.64–0.80), combined CRP with IgG and IgA was 0.73 (95% CI, 0.64–0.80). We found that CRP combined with IgG, IgA and IgG increased the diagnostic accuracy comparing with CRP alone in bacteriological negative tuberculosis patients (P<0.05). The AUC of CRP (0.691, 95% CI, 0.607–0.768) was higher than those combined CRP with IgG (0.684, 95% CI, 0.599–0.761) and combined CRP with IgG and IgA (0.686, 95% CI, 0.601–0.763), without statistical differences (P>0.05, P=0.716, 0.805, 0.880) in bacteriological positive group.

We showed the comparisons of diagnostic accuracy of CRP alone, two combined assays in bacteriological positive group and bacteriological negative group in Figure 4. All three assays including CRP alone, combined assay with CRP and IgG, combined assay with CRP, IgG and IgA showed no statistical differences between two groups (P=0.068, 0.530 and 0.515).

Discussion
Our study found that CRP demonstrated limited diagnostic value, even when combined with IgG and IgA, in the diagnosis of pulmonary tuberculosis. Both combined assays could increase the diagnostic accuracy in bacteriological negative group than CRP alone.
Despite the increase in pulmonary tuberculosis notifications, there is still a significant gap between the number of new cases reported (7 million) and the estimated 10 million cases (ranging from 9–11.1 million) in 2018 (1). Rapid case detection and early treatment are the most effective methods to prevent the spread of pulmonary tuberculosis and reduce its burden (16). Previous studies had proved the association between pulmonary tuberculosis and CRP, IgG and IgA, consistent with the potential diagnostic value of CRP, IgG and IgA (9,17,18). We did find that CRP values and IgG values had statistical differences between pulmonary tuberculosis group and control group. Besides, we compared the equality of values between the bacteriological positive pulmonary tuberculosis and bacteriological negative pulmonary tuberculosis through Wilcoxon rank sum tests and there were no significant statistics differences of these two groups. On the contrary, other studies had found that higher CRP values were significantly related to higher mycobacterial load, increased frequency of pulmonary tuberculosis transmission and higher risk of death (14,19,20).
It has been shown previously that when cut-off points of CRP were implemented to assess their utility in pulmonary tuberculosis diagnosis, sensitivity decreased and specificity increased when CRP values were raised in turn (19,21), which was consistent with our results. We estimated the values of sensitivity, specificity, positive likelihood ratio (+LR) and negative likelihood ratio (−LR) of CRP for different CRP cut-off points in three groups. The values of sensitivity were higher than 50% when CRP >1 mg/L in three groups, then it went down significantly with raising CRP cut-off values. When CRP >5 mg/L, the values of specificity were quite high, all above 90%, meaning that CRP might be more useful for reducing misdiagnosis rate.
We further compared the diagnostic values of CRP alone, combined assay with CRP and IgG, combined assay with CRP, IgG and IgA in different groups. All of them could potentially discriminate between pulmonary tuberculosis patients and healthy population, but the accuracy of pulmonary tuberculosis diagnosis was not high. For bacteriological negative group, both combined assays could increase the diagnostic accuracy than that of CRP alone. Although the present studies had proved the diagnostic values of blood-based biomarkers, the complexity reiterates the complexity and heterogeneity of pulmonary tuberculosis pathology and host-pathogen interactions (22,23). In the bacteriologically lower respiratory tract infection, the detection of CRP is neither sufficiently sensitive to rule out, nor sufficiently specific to rule in (24).
However, there were a few limitations. Firstly, there may be bias in the selection of control group resulting in inaccurate association. Secondly, the CRP values were relatively lower than other studies and the cut-off points for pulmonary tuberculosis diagnosis were lower. One reason is different regions and participants may influence the value of CRP.
In conclusion, our study found that all three assays including CRP alone, combined assay with CRP and IgG, combined assay with CRP, IgG and IgA maybe potentially discriminated between pulmonary tuberculosis patients and healthy population. Both combined assays could increase the diagnostic accuracy in bacteriological negative group than CRP alone. Limited application of serodiagnostic assays suggested the need of evaluating different diagnostic strategies.
Acknowledgments
Funding: The study was supported by “Postgraduate Research & Practice Innovation Program of Jiangsu Province” (grant number KYCX19-1131) and The National Twelfth Five-year Mega-Scientific Projects of infectious diseases of China (grant number 2018ZX10715002).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jphe-20-38
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jphe-20-38
Peer Review File: Available at http://dx.doi.org/10.21037/jphe-20-38
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe-20-38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was considered part of routine public health surveillance and, due to this, was considered exempt from ethical approval. All data were de-identified prior to use, and identifying information was not available. Written informed consent was obtained from all eligible TB patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Global tuberculosis report 2019. 2019. Available online: https://www.who.int/tb/publications/global_report/en/. Accessed 17 October 2019.
- Granich R. Is the global tuberculosis control strategy too big to fail? Lancet 2018;392:2165. [Crossref] [PubMed]
- World Health Organization. Implementing tuberculosis diagnostics: A policy framework. 2015. Available online: https://www.who.int/tb/publications/implementing_TB_diagnostics/en/. Accessed 27th April 2015.
- Floridia M, Ciccacci F, Andreotti M, et al. Tuberculosis Case Finding with Combined Rapid Point-of-Care Assays (Xpert MTB/RIF and Determine TB LAM) in HIV-Positive Individuals Starting Antiretroviral Therapy in Mozambique. Clin Infect Dis 2017;65:1878-83. [Crossref] [PubMed]
- Mohd Hanafiah K, Garcia M, Anderson D. Point-of-care testing and the control of infectious diseases. Biomark Med 2013;7:333-47. [Crossref] [PubMed]
- Shapiro AE, Hong T, Govere S, et al. C-reactive protein as a screening test for HIV-associated pulmonary tuberculosis prior to antiretroviral therapy in South Africa. AIDS 2018;32:1811-20. [Crossref] [PubMed]
- Gao X, Guo X, Li M, et al. Interleukin 8 and Pentaxin (C-Reactive Protein) as Potential New Biomarkers of Bovine Tuberculosis. J Clin Microbiol 2019;57:e00274-19. [Crossref] [PubMed]
- Castell JV, Gomez-Lechon MJ, David M, et al. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology 1990;12:1179-86. [Crossref] [PubMed]
- Mohd Hanafiah K, Garcia ML, Anderson DA. An Observational Case-Control Study to Determine Human Immunodeficiency Virus and Host Factor Influence on Biomarker Distribution and Serodiagnostic Potential in Adult Pulmonary Tuberculosis. Trop Med Infect Dis 2019;4:57. [Crossref] [PubMed]
- Ziegenbalg A, Prados-Rosales R, Jenny-Avital ER, et al. Immunogenicity of mycobacterial vesicles in humans: identification of a new tuberculosis antibody biomarker. Tuberculosis (Edinb) 2013;93:448-55. [Crossref] [PubMed]
- Davidow A, Kanaujia GV, Shi L, et al. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect Immun 2005;73:6846-51. [Crossref] [PubMed]
- Abebe F, Belay M, Legesse M, et al. IgA and IgG against Mycobacterium tuberculosis Rv2031 discriminate between pulmonary tuberculosis patients, Mycobacterium tuberculosis-infected and non-infected individuals. PLoS One 2018;13:e0190989 [Crossref] [PubMed]
- Hussain MI, Ahmed W, Nasir M, et al. Immune boosting role of vitamin E against pulmonary tuberculosis. Pak J Pharm Sci 2019;32:269-76. [PubMed]
- Yoon C, Chaisson LH, Patel SM, et al. Diagnostic accuracy of C-reactive protein for active pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2017;21:1013-9. [Crossref] [PubMed]
- Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [Crossref] [PubMed]
- Ji Y, Cao H, Liu Q, et al. Screening for pulmonary tuberculosis in high-risk groups of diabetic patients. Int J Infect Dis 2020;93:84-9. [Crossref] [PubMed]
- Niki M, Yoshiyama T, Miyamoto Y, et al. Longitudinal Evaluation of Humoral Immunity and Bacterial and Clinical Parameters Reveals That Antigen-Specific Antibodies Suppress Inflammatory Responses in Active Tuberculosis Patients. J Immunol Res 2018;2018:4928757 [Crossref] [PubMed]
- Yan ZH, Yi L, Wei PJ, et al. Evaluation of panels of Mycobacterium tuberculosis antigens for serodiagnosis of tuberculosis. Int J Tuberc Lung Dis 2018;22:959-65. [Crossref] [PubMed]
- Lawn SD, Kerkhoff AD, Vogt M, et al. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis 2013;17:636-43. [Crossref] [PubMed]
- Ciccacci F, Floridia M, Bernardini R, et al. Plasma levels of CRP, neopterin and IP-10 in HIV-infected individuals with and without pulmonary tuberculosis. J Clin Tuberc Other Mycobact Dis 2019;16:100107 [Crossref] [PubMed]
- Bedell RA, van Lettow M, Meaney C, et al. Predictive value of C-reactive protein for tuberculosis, bloodstream infection or death among HIV-infected individuals with chronic, non-specific symptoms and negative sputum smear microscopy. Trop Med Int Health 2018;23:254-62. [Crossref] [PubMed]
- Lenaerts A, Barry CE 3rd, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev 2015;264:288-307. [Crossref] [PubMed]
- Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers 2016;2:16076. [Crossref] [PubMed]
- van der Meer V, Neven AK, van den Broek PJ, et al. Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ 2005;331:26. [Crossref] [PubMed]
Cite this article as: Liu Q, Yang D, Ji Y, Martinez L, Wang J, Shi X, Zeng Y, Lu W. Diagnostic value of C-reactive protein, IgG, and IgA for detection of pulmonary tuberculosis: a case-control study from Eastern China. J Public Health Emerg 2020;4:30.