
SmartHIV Manager: a web-based computer simulation system for better management of HIV services
Introduction
The advent of highly active antiretroviral therapy (HAART) has been the cornerstone of human immunodeficiency virus (HIV) care over the last two decades. This has enabled people living with HIV/acquired immunodeficiency syndrome (AIDS) (PLWHA) to live a relatively normal life like the general population. Despite these life-changing developments, other challenges remain or have emerged which include management of co-morbidities, polypharmacy, ageing, and concurrent management of other chronic diseases.
To establish the needs of those involved in the management of HIV care, we conducted a survey to identify the gaps in technology which require support in HIV/AIDS management. We targeted a convenience sampling of HIV practitioners actively involved in HIV/AIDS management. We received a total of 45 responses from experts (100% response rate) around the world. According to the survey (see Table 1), personalized clinical decision making; resource planning, management and service redesign; co-morbidity, co-infection and resistance management; and profiling antiretroviral therapy (ART) regimen are the features with the highest level of importance amongst these respondents, which if addressed, it could assist with the challenges they are currently facing.

Full table
Despite the vast HIV research output over the last 40 years, there are no known user-friendly web-based systems within a single platform that can assist the needs expressed by experts and specialists. To address the needs of service managers and decision makers, we developed the SmartHIV Manager. Despite the availability of a widening array of effective HIV prevention tools and methods and a massive scale-up of HIV treatment in recent years, the joint United Nations programme on HIV/AIDS (UNAIDS) cautions there has been unequal progress in reducing new HIV infections. SmartHIV Manager supports this global commitment to stopping new HIV infections and ensuring that everyone with HIV has access to HIV treatment.
SmartHIV Manager
SmartHIV Manager models the HIV care continuum, by capturing in detail the sequence of steps a person with HIV takes from diagnosis through receiving treatment until his or her viral load is suppressed to undetectable levels and regular monitoring. It is an easy-to-use web-based system developed to assist key decision makers for better management of HIV services. It is based on simulating an entire patient pathway within a HIV service, from initial referral to discharge, including diagnostics, treatment, monitoring, counselling, suppression (virologic failures), adherence counselling and death.
Some of its features enable users to forecast activity and service use over an extended period of time; predict and optimise required resources (e.g., doctors, nurses, rooms); establish how to achieve UNAIDS 90-90-90 target (1). In real-time, users can compare current practices versus potential scenarios and generate a wide range of results to assess the impact of change on service demand and utilization, prevention, budgeting and financial planning, resource planning and management, and cost-effectiveness analysis.
SmartHIV Manager is a platform that will enable key decision-makers evaluate decision options accurately. More importantly, it has the ability to test before implementing in practice to avoid the trap of “doing things and hoping for the best”, thus the opportunity to generate efficiencies in the HIV patient pathway and improve performance.
Methods
Discrete event simulation (DES) is a technique used to depict a system within a computer simulation environment to observe its behaviour and state changes over time (2). It is widely used to model patient pathway and operational aspect of the systems (3-7). DES is a well-established approach by the healthcare community, for its adaptability, suitability, and scalability (8).
We developed a DES model to capture individual patient’s footsteps from initial referral to HIV clinic, treatment and monitoring over a period of time. The model reflects all the activities and resources utilized at each visit like diagnostic activities, ART treatment, disease progression, counselling and intensified adherence counselling. First is the conceptualisation stage, where a comprehensive view of an entire patients’ pathway within HIV care is established to capture all the uncertainties and variations (e.g., demand, resource utilization, patient outcomes). If the system is captured to a certain degree of accuracy and detail, endless “what if” scenarios can be examined to assess the impact of change, not just on one aspect of the system, but its knock-on effect on other parts (either directly or indirectly). For example, when assessing the impact of preventive measures [e.g., pre-/post-exposure prophylaxis (PrEP/PEP)], why just focus on the number of averted patients and its cost-effectiveness. What about the impact of prevention on service demand, utilization, personnel requirements, budgeting and financial planning? When solving such problems, a myopic view can lead to inaccurate (incomplete) findings, and thus a sub-optimal solution.
Therefore, the conceptualization process was carried out with services at several countries, within Nigeria, United Kingdom, South Africa, and Kenya. The team is made up of HIV physicians, HIV service managers, policy makers and HIV researchers. Therefore, the pathway was captured to a sufficient level of details such that it is applicable to a wide range of HIV services around the world. The World Health Organization (WHO) guidelines formed the baseline pathway, where necessary adjustments were made to ensure individual service provision of countries is considered (9).
A typical HIV patient pathway around the world is made up of five components, namely prevention, diagnosis, treatment, monitoring and disease progression. Necessary resources were attached for each patient at each stage of the pathway, including a clinic room, a physician, a counsellor, nurse(s), a pharmacist, lab technicians for diagnostic testing purposes and community/social services. Individual patient attributes within the pathway of care were also captured, e.g., patient type (naïve or Tx experienced), sex, age group, pregnancy, ART treatment class (first/second line), HIV suppressed/failed, and viral load groups. Depending on patient attributes, frequency of monitoring per year, clinical outcomes, adherence/non-adherence, comorbidities and co-infections were modelled accordingly. All essential information was captured to ensure the simulation model depicted a real-life HIV service as much as possible. The model was developed using simulation software Simul8.
Statistical analysis
The main analysis was performed with the input data, which is collected to capture the pathway of the service. To test and validate our model, a data template was created to collect the input parameters from three different HIV centres, two in Nigeria and one in Kenya. A total of 93 input parameters were established covering the entire HIV pathway of care. Where data was not available, we resorted to the literature, expert opinions and online resources. Inputs include demand, pathway related parameters (e.g., mix of resources, treatment times, number of visits etc.), treatment effectiveness (e.g., viral load failure rate, probability of prevention), and costs.
Country level HIV treatment costs for “Budgeting and Financial Planning” purposes were collected through an exhaustive review of the literature covering 139 countries globally. Costing data was collected in US dollars or Euro for each country, which included first-line/second-line ART costs per person per year; laboratory cost per person per year, and overhead and personnel cost per person per year. Overhead costs included facility utilities (water, electricity, other), facility support staff (guards, cleaners), facility-level administrative staff, general consumables/other supplies at the site, transport/monthly running costs of vehicles (if used for ART).
Tool design
An animated interface in a 2D isometric illustration was designed with necessary control buttons, so that users could change the input parameters accordingly, thus service-specific (see Figure 1). SmartHIV Manager is a web-based platform, which can be used in real-time, enabling users to analyse the impact of alternative scenarios and interventions as and when needed. Dashboards were developed using Java Spring boot, communicating with the simulation model, generating a comprehensive set of outputs in a simple, graphically appealing and easy to understand format. Our platform is very responsive which can be used on any device, i.e., mobile phones, tablet and desktop. The dashboards are made up of Service Demand and Utilization, Prevention, UNAIDS 90-90-90, Human Resource Management & Planning, and Budgeting and Financial Planning.
As shown in Figure 1, SmartHIV Manager has a simple user interface with a submerged highly complex simulation structure at the back end. The complex part is not visible to ordinary users as key decision-makers are not interested in the technical details of the model. SmartHIV Manager runs over a 5-year period (but not limited to), comparing the “Current” practice against a “Scenario”. Exhaustive set of exportable outputs (in the form of PDF and Excel) are available in the dashboards (see Figure S1) with graphical and numerical results.
As the simulation runs, the front interface is animated, enabling users to interact and communicate, thus the opportunity to observe the behaviour of their system under various conditions. For example, HIV patients do not only flow through instantaneously, but also spend time and consume healthcare resources. DES provides insights on cause-and-effect relationship between demand and capacity. As a result, patients might wait if the resource is not available at that moment, which can be seen in the animation.
Verification and validation of a model is critical for accountability and to ensure the results are robust, reliable and accurate. This is a vital process in any simulation model building, where all stakeholders, made up of clinicians, service managers and nurses were engaged in every step of development, so that the model can be verified and validated. We also compared the simulation outputs with real-world outputs, which was 5% either side of the expected result, suggesting that the model is fit for purpose.
Results
We demonstrated how SmartHIV Manager can be used in practice for a single centre in Nigeria. However, the system is generic and can be used by any HIV service provider globally. Amongst many features and functionalities, we demonstrated the impact of increasing prevention measures (Scenario 1–3) and increasing the number of people on treatment to reach UNAIDS 90-90-90 target (Scenario 4).
The service in this study currently PrEP and PEP for around 45 patients annually. There is a 20% and 80% split in the number of patients offered PrEP and PEP, respectively. The number of people offered PrEP and PEP is increased from 45 to 100 (Scenario 1), 250 (Scenario 2), and 500 people (Scenario 3) with a 50/50 split. On the other hand, in Scenario 4 the number of naïve patients increased from 100 to 120 per year (with a 5% growth rate).
Model outputs and dashboard
The model was run for 5.5 years with a warm-up period of 6 months. A wide range of outputs were generated, e.g., demand, activity, ART treatment, and number of averted cases due to prevention, UNAIDS 90-90-90 target values. Then, visual graphics are produced and transferred to the results dashboard. These steps are fully automated so the results can be easily interpreted via the results dashboard (see Figure S1). The number of averted cases due to PrEP/PEP have remarkably increased throughout the years (see Figure 2), e.g., from 1.75 people (baseline) to 40 people (Scenario 3) in total for PrEP.
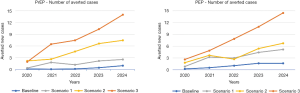
The number of monitoring visits due to prevention is expected to increase dramatically. For instance, in the case of Scenario 3, the number of visits during the 5-year period is expected to increase from 535 and 562 visits (baseline) to 15,359 and 4,140 visits (Scenario 3) for PrEP and PEP, respectively (see the top part of Table 2). PrEP visits increased sharply due to following reasons. Firstly, the proportion of people on PrEP has increased from 20% (baseline) to 50% in all the scenarios. Secondly, patients on PrEP are not discharged unless they drop out (around 5%). Therefore, the number of patients in the PrEP cumulatively increases, whereas patients on PEP visit the service three times (within 6 months) then discharged.
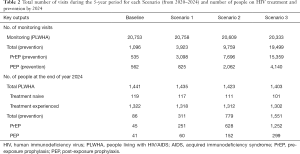
Full table
On the other hand, a noticeable reduction is expected in the number of monitoring visits by PLWHA, i.e., from 20,753 visits over the 5 years (baseline) to 20,333 (Scenario 3). This is due to the decrease in the number of averted cases (i.e., naïve patients). The impact of the scenarios can be better evaluated by inspecting the difference in the number of PLWHA and prevention programmes (see the bottom part of Table 2). The number of PLWHA on treatment decreased to 1,403 (Scenario 3) from 1,441 (baseline), whereas very little difference between baseline and Scenario 1 (6 patients). This shows that even a small increase in prevention measures has made a positive impact.
UNAIDS 90-90-90
Scenario 4 assesses the situation of the service against UN 90-90-90 target (now and by 2025). ‘90-90-90 targets’ were set by the UNAIDS and the WHO (1). The targets aim to diagnose 90% of all HIV positive people, provide ART for 90% of those diagnosed and achieve viral suppression for 90% of those treated, by 2020.
As of 2020, the service currently has 1,079 diagnosed patients within their catchment area, which should be at least 1,349 patients according to the UN target (see Table 3). The service provides ART treatment for all diagnosed patients (1,079 patients), however the number should be 1,214 patients. The service at present has 970 patients virally suppressed, whereas this figure is expected to be around 1,092 (if the third 90% in the UN target was achieved).
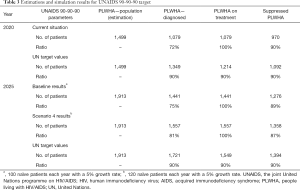
Full table
The service has 100 naïve patients annually with a 5% annual increase. According to the estimations, the service should diagnose and provide treatment to at least 120 naïve patients each year (with a 5% annual increase) to reach the UN target by 2025. Thus, the model is run accordingly for Scenario 4.
As a result of the increase in demand (see Table 3), the number of PLWHA in 2025 is expected to increase 1,557 (Scenario 4) which is higher than UN target (1,549 patients). The service is slightly below the target in Scenario 4 in terms of PLWHA with viral suppression (1,358 vs. 1,394 patients), 87% vs. 90%. However, more PLWHA are supressed (1,358 patients) against the baseline number (1,276 patients). Lastly, the number of monitoring visits by PLWHA over the 5 years increased from 20,711 (baseline) to 22,379 (Scenario 4).
Discussion
Critical to the UNAIDS target for 2020 is the goal 90-90-90 (10). A major threat to the actualization of this goal is the inefficiency in treatment management with development of HIV drug resistance, often associated with poor adherence (11). Towards achieving this goal, there is a need for an efficient system to regularly assess, teleguide and troubleshoot along the treatment cascade. The developed system provides a web-based technology to assess the service performance aiming to improve the treatment of PLWHA. A wide range of outputs inform key decision makers (e.g., manager, clinician) about the potential impact of improving certain aspects of their services.
Achieving the UNAIDS target, especially in developing countries, could significantly reduce the overall HIV/AIDS burden and mortality. This target was aimed at eliminating HIV infection as a public health threat by 2030 which is anchored on the connected tripod of HIV diagnosis, linkage as well as retention in care and viral load suppression. In a similar study in India, Maddali et al. estimated a reduction in new HIV cases of about 50% and about 60% in AIDS-related mortalities from their model using the same target (12). Ultimately, an improvement in the global HIV/AIDS statistics using these indicators would prevent further HIV transmission, even in the high-risk population, and enhance the quality of life among PLWHA. It is however important for the individual countries to identify their peculiarities and achievements for the proper implementation of policies towards building on the currently recorded successes due to the variations in the barriers across the different country’s income strata (13).
SmartHIV Manager generates a concrete evidence for improvement in the management strategies to upscale the standard and process of HIV care. Therefore, the users will better understand the required interventions necessary in the care system, planning and policy formulation directed at their local HIV population. For example, in Scenario 4 (reaching UNAIDS 90-90-90 target), the findings indicate that the service needs to increase their naive demand (i.e., new patients) by 20% to reach the target by 2025. The impact of the increase in demand on service outcomes (i.e., the number of monitoring visits) is clearly shown. Therefore, the findings will guide service managers for better planning and resource allocation in short to medium term.
To the best of our knowledge, there is no such web-based system for enabling better management of PLWHA for key decision makers. Most emphasis by the previously developed support were focused on clinicians for therapeutic inventions like drug interactions in HIV medicine. Our web-based model was developed to fill the gaps by going beyond individual patients’ care and predicting the facility performance for efficient programmatic management.
The creation of the President’s Emergency Plan for AIDS Relief (PEPFAR) in 2003 revolutionized the management of HIV/AIDS with its great impact in sub-Saharan Africa (sSA). However, this funding by PEPFAR is dwindling and most of the countries in sSA are yet to take ownership of the programme. In order not to forfeit the gains of many years, the adoption of innovative technologies by agencies and countries in the preventive drive of HIV/AIDS becomes sacrosanct. The predictive function of this technology would be needed for global health planning and policy development where resources can be properly deployed and monitored.
Our application has far reaching benefits in health planning and policy making especially for HIV control on an individualized basis which could help reduce the spread of the disease. Health facilities and regional HIV programmes could also use this technology to assess their settings and improve the management of patients, and for decision making. SmartHIV Manager will make possible the achievement of the global commitment to stopping new HIV infections and ensuring that everyone with HIV has access to HIV treatment.
Conclusions
A web-based computer simulation system, SmartHIV Manager, that integrates the summation of the care processes in HIV management is urgently needed for the effective evaluation and prediction of possible outcomes towards optimal patients’ care. This novel web-based platform would enhance HIV/AIDS control and evidence-based patients care for a holistic patient management.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jphe-20-133
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe-20-133). Dr. APK serves as an unpaid editorial board member of Journal of Public Health and Emergency from Jun 2019 to May 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS, 2014.
- Pidd M. Tools for thinking: modelling in management science. London: Wiley, 2009.
- Rauner MS, Brailsford SC, Flessa S. Use of discrete-event simulation to evaluate strategies for the prevention of mother-to-child transmission of HIV in developing countries. J Oper Res Soc 2005;56:222-33. [Crossref]
- Duguay C, Chetouane F. Modeling and improving emergency department systems using discrete event simulation. Simulation 2007;83:311-20. [Crossref]
- Chemweno P, Thijs V, Pintelon L, et al. Discrete event simulation case study: Diagnostic path for stroke patients in a stroke unit. Simul Model Pract Theory 2014;48:45-57. [Crossref]
- Lebcir R, Demir E, Ahmad R, et al. A discrete event simulation model to evaluate the use of community services in the treatment of patients with Parkinson's disease in the United Kingdom. BMC Health Serv Res 2017;17:50. [Crossref] [PubMed]
- Pan F, Reifsnider O, Zheng Y, et al. Modeling clinical outcomes in prostate cancer: application and validation of the discrete event simulation approach. Value Health 2018;21:416-22. [Crossref] [PubMed]
- Katsaliaki K, Mustafee N. Applications of simulation within the healthcare context. J Oper Res Soc 2011;62:1431-51. [Crossref] [PubMed]
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013. Available online: https://www.who.int/hiv/pub/guidelines/arv2013/en/ (accessed 02 July 2020).
- Granich R, Gupta S, Hall I, et al. Status and methodology of publicly available national HIV care continua and 90-90-90 targets: a systematic review. PLoS Med 2017;14:e1002253 [Crossref] [PubMed]
- Revell AD, Wang D, Wood R, et al. An update to the HIV-TRePS system: the development and evaluation of new global and local computational models to predict HIV treatment outcomes, with or without a genotype. J Antimicrob Chemother 2016;71:2928-37. [Crossref] [PubMed]
- Maddali MV, Gupta A, Shah M. Epidemiological impact of achieving UNAIDS 90-90-90 targets for HIV care in India: a modelling study. BMJ Open 2016;6:e011914 [Crossref] [PubMed]
- Karatzas N, Peter T, Dave S, et al. Are policy initiatives aligned to meet UNAIDS 90-90-90 targets impacting HIV testing and linkages to care? Evidence from a systematic review. PLoS One 2019;14:e0216936 [Crossref] [PubMed]
Cite this article as: Adeyemi S, Demir E, Yakutcan U, Adeoti A, Kengne AP, Kayode GA, Aliyu A, Idika N, Isichei C. SmartHIV Manager: a web-based computer simulation system for better management of HIV services. J Public Health Emerg 2021;5:13.



