
Technical specifications for nucleic acid test sampling sites for prevention and control of COVID-19 pandemic in Jiangsu Province
Introduction
The purpose of this article is to share the strategies and guidelines adopted for setting up the layout and highlight the basic requirements at nucleic acid testing (NAT) sampling sites to prevent and control the coronavirus disease 2019 (COVID-19) pandemic in Jiangsu Province. The novel omicron coronavirus outbreak since March 2022 has led to adopt effective epidemic prevention and control measures in all parts of Jiangsu Province. The transmissible capacity of the omicron variant strain is significantly higher than that of other coronavirus variants. Further, it also has a short incubation period and a high viral load, posing new challenges for epidemic prevention. As one of the usual diagnostic methods for suspected coronavirus cases is NAT in a suspected area which can provide evidence for early detection, early reporting, early isolation, and early treatment of COVID-19. In order to speedily control the source of infection, effectively block the chain of transmission, and prevent the spread of the epidemic, the Jiangsu provincial government quickly launched large-scale NAT sites in local communities, and all cities quickly rolled out nucleic acid screening for all staff. In the initial stage of the epidemic, Jiangsu province quickly set up the medical teams for nucleic acid test collection to carry out large-scale nucleic acid test sampling in various communities. This paper retrospectively analyzed the team building, material management, sampling process, personal protection, and environmental disinfection of the medical team, and discussed the standardized management and process optimization of nucleic acid test sampling sites during the COVID-19 outbreak, to achieve the goal of reducing cross-infection rate.
Methods
Basic requirements
Organizational management
Major responsibilities in COVID-19 prevention and control should be strictly fulfilled. An organizational system for COVID-19 emergency prevention and control should be established and led by the person-in-charge of sampling sites. A leading group for COVID-19 prevention and control also needs to be set up, so that tasks associated with COVID-19 prevention and control can be assigned to specific departments or personnel.
Development of plans
The sampling sites need to build an early warning system, and develop contingency plans and targeted action plans for outbreak periods and non-outbreak periods based on the etiological characteristics of the novel coronavirus, sources of infection, routes of transmission, susceptible populations, and the quality of diagnosis and treatment.
Material support
Supply of materials and funds necessary for COVID-19 prevention and control needs to be guaranteed. Material supplies, such as personal protective equipment, disinfectants, disinfecting apparatus, and medical equipment, need to be well prepared in response to the epidemic.
Epidemic management
The sampling sites should collaborate with the health departments in managing the spread of COVID-19 when suspected, confirmed, or asymptomatic cases of COVID-19 are reported.
Prevention and control measures
Sampling sites
Sampling sites should be set up as separate structures independent of other buildings to provide relatively independent spaces and facilitate cleaning and disinfection. If the above requirements cannot be satisfied, temporary sampling sites with a roof above and a four-sided shield around can be erected in outdoor open spaces relatively far from crowded areas. Warning signs should be installed at sampling sites. Proper ventilation with outside air should be maintained. Clear signage should also be put up to provide instructions for specimen collection to the public.
Waiting zones and sampling zones should be established at sampling sites. One sampling room (zone) should be dedicated for one individual at a time during the sampling process. Sampling spaces at sampling sites should be properly arranged based on the number of people. Waiting areas should be arranged in a coordinated way and special staff should be assigned to manage the flow of people so that individuals queuing for sampling can maintain a minimum safe distance of 1 m between one another in the waiting area.
Before entering the sampling site for specimen collection, visitors should cooperate with the staff by proactively showing their ID cards and their personal health information codes of Jiangsu, getting their temperatures taken, and filling in the personal information form.
Sampling sites should be designated for individuals who are assigned green or yellow health codes. For individuals who are assigned green health codes, 10-sample pool testing should be performed. For individuals who are assigned yellow health codes, three nucleic acid amplification tests (NAATs) (3-sample pool testing) need to be performed within 1 week. The interval between the first and the second test should be 24 h and the third test needs to be performed on the 6th day.
Sampling sites should be partitioned and sampling time slots should be allocated in an orderly manner based on the area served and its population density.
Individuals waiting for sampling should wear masks and stay away from the sampling zone. At their turn, they can enter the sampling zone individually. Any close contact with people other than the sampling staff should be avoided during the whole process.
The sampling sites should prepare facial tissues in advance. The facial tissues can be used to cover the mouth and the nose when sneezing or coughing during the collection of respiratory tract specimens, including nasal swabs and throat swabs.
If yellow or red health code-assigned individuals are found at sampling sites designated for green health code-assigned individuals, the leading group for COVID-19 prevention and control at the corresponding sampling site should immediately place these individuals with non-green codes in quarantine. Individuals with yellow codes should be sent to the corresponding sampling sites for NAATs with dedicated transport vehicles. Individuals with red codes should be reported to the local centers for disease control and prevention in a timely manner. Individuals with non-green codes are absolutely forbidden from leaving the sampling sites on their own by public transport.
Results of NAATs should be uploaded to the Jiangsu Health Code (SKM) (personal health information code of Jiangsu, refers to a sequence of numbers or letters that is bound to the online ID credential of the resident. The code indicates that the user authorizes other people or organizations to temporarily access specific personal health information. QR codes are generally used as a storage medium for health codes. Personal health digital QR codes are generated in real time based on the health data of the declared individual and comparisons with other relevant data. The three-colored QR codes—red, yellow, and green—indicate high, moderate, and low risk of infection, respectively) or other information systems within 24 h for convenience of checking the results.
Sampling staff
Training contents tailored for different staff members should be developed based on their job responsibilities, so that they can master the knowledge, methods, and skills needed for COVID-19 prevention and control.
All sampling staff at the sampling sites should be fully vaccinated against COVID-19. Collection of specimens from the sampling staff should be completed by the medical institutions they are employed with.
A health monitoring system should be established for the staff. Staff health status should be recorded daily. In case of suggestive symptoms, medical care should be sought as soon as possible.
All staffs need to have their temperature taken before entering the sampling site. The SKM (must be green-colored) and proof of a negative NAAT result (within 48 h) need to be presented.
Specimen collectors should be qualified professionals who have received biosafety training and are equipped with the technical skills in specimen collection and personal protection. Specimen collectors should try their best to position themselves upwind of the individual from whom the specimen is taken and should not work continuously for more than 2 h.
Specimen collectors should wear work outfits, disposable working caps, double-layered gloves, disposable medical protective clothing, medical protective masks or powered air purifying respirators, goggles or protective face shields, work shoes or rubber shoes, and waterproof boot covers. In case of contact with the patient’s nasopharyngeal secretions, the outer latex gloves should be promptly replaced.
Transport of specimens
After the specimens are collected, the outer surface of the specimen containers should be wiped or sprayed with 1,000 mg/L chlorine-containing disinfectants. The specimen containers should then be placed inside dedicated self-sealing specimen bags with biosafety warning signs. After the outer surface of the self-sealing bags are disinfected using 1,000 mg/L chlorine-containing disinfectants, these bags should be placed inside sealed boxes dedicated to specimen transport. The outer surface of the transport boxes should then be disinfected using 1,000 mg/L chlorine-containing disinfectants.
Specimens should be transported to the laboratories by professionals who have received biosafety training. All staff members involved in transport of specimens should wear caps, disposable surgical masks, gloves, and isolation gowns. During transport, stability of transport boxes should be ensured and violent jolts should be avoided.
Specimen receivers at the laboratory should disinfect the transport boxes using 1,000 mg/L chlorine-containing disinfectants before opening the boxes. After the self-sealing bags are disinfected with 1,000 mg/L chlorine-containing disinfectants, they should be opened in a biological safety cabinet and the specimens are taken out from the bags. Specimen information should then be checked in detail and the specimen handover recorded.
Personal protection
All staff undertaking on-site work at the sampling sites should improve hand hygiene practices. Effective alcohol-containing fast-drying hand sanitizers can be used. Under special conditions, hand sanitizers containing chlorine or hydrogen peroxide can also be used. If the contaminants are visible by eye, hands should be cleaned with running water and hand sanitizers before disinfecting them. Specimen collectors should use fast-drying hand sanitizers to disinfect their hands after a specimen is collected from one patient. Gloves that are visibly contaminated should be immediately changed. Cross infections should be prevented by strictly enforcing the predetermined procedures: sanitize the hands and change the needle, the tourniquet, and the sterile towel after each specimen collection and ensure that specimens are taken from one patient at a time. Upon completion of the work, wash hands with running water and hand sanitizers before disinfecting them.
When the skin is contaminated, the contaminants should be immediately removed. Disposable water absorbent materials dipped into the 0.5% iodine or hydrogen peroxide disinfectant should then be used to wipe and disinfect the contaminated skin for over 3 min before the skin is rinsed with clean water. Mucosae should be rinsed with large volumes of normal saline or rinsed and disinfected with 0.05% iodine disinfectant.
All staff in sampling sites for yellow health code-designated individuals should wear protective clothing.
Medical waste
Upon completion of specimen collection, the sampling staff should write the name of the sampling site and the date of delivery on the sealing labels (infectious medical waste labels). Medical wastes should be packaged in double-layered yellow medical waste bags secured with gooseneck knots and layer-by-layer sealing. The waste bags should then be properly labelled.
Medical waste bags should be sealed when three-quarters full. The sampling staff should then fill in the medical waste registers before handing over the wastes to the medical waste collectors. Signatures of both parties should be provided.
Medical waste collectors should bring along the medical waste registers, place the medical waste in the transport boxes, and transport the boxes to the temporary storage facilities for medical waste. The staff at the temporary storage facility should then weigh the transport boxes and complete the handover process. Signatures of both parties should be provided.
Transport vehicles should be disinfected with 1,000 mg/L chlorine-containing disinfectants after completion of the transport. Allow the disinfectant to act for 30 min before wiping the disinfectant off with clean water.
Environmental disinfection
It is recommended that the sampling sites install man-machine coexistence dynamic air disinfection machines to continuously disinfect air.
Ultraviolet light can be used to disinfect air in rooms when empty, with irradiance of ≥70 µW/cm2 and >1 h exposure time; 500 mg/L chlorine dioxide, 30 g/L hydrogen peroxide, or 2,000 mg/L peroxyacetic acid disinfectants can also be applied as ultra-low volume (ULV) sprays or aerosol sprays (20 mL/m3). The disinfectant should be allowed to act for 30 min or the room exposed to vapor-phase hydrogen peroxide for over 30 min. Environmental disinfection should be performed at least twice daily.
In case of no visible contaminant, the surface of the ground, the tables, the chairs, and other indoor objects can be wiped with chlorine-containing disinfectants (500–1,000 mg/L) or disinfectants containing 500 mg/L chlorine dioxide before the residue is wiped off with clean water. The disinfection procedure should be performed at least twice daily.
If the surface of the ground, the walls, or the abovementioned objects are visibly contaminated by blood, body fluids, or secretions, the visible contaminant in liquid state should be immediately removed with disinfecting wipes or disposable water absorbent materials (e.g., absorbent papers, gauze, etc.) dipped into disinfectants containing 10,000 mg/L effective chlorine. Disinfectants containing 500 mg/L chlorine dioxide should then be used to spray or wipe the contaminated surface.
At sampling sites designated for individuals assigned yellow health codes, frequently used public restrooms should be disinfected every 2 h.
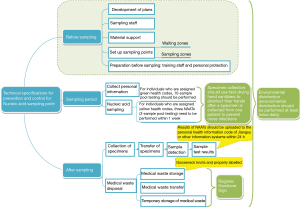
Flow chart
A flow chart as shown in Figure 1, is used to summarize the procedures for adopting technical specifications at NAT sampling sites.
Results
From March 5 to May 4, 2022, more than 220,000 NAT sampling sites have been set up, and the number of NAT samples have been exceeded 1.5 billion since the omicron COVID-19 virus broke out. In the meantime, 2,877 confirmed cases have been detected through NAT sampling, accounting for 80.47 percent of the total confirmed cases (n=3,575) reported in Jiangsu Province. None of the suspected novel coronavirus infection cases were missed or misdiagnosed at NAT sampling sites in Jiangsu Province. No cross infection was reported in any medical staff members of NAT sampling sites. Effective NAT sampling plays an important role in quickly identifying and controlling the source of infection, efficiently blocking the chain of transmission, and preventing the spread of the epidemic.
Discussion
Cross-infection and epidemic spread can be effectively avoided through the joint cooperation of multiple departments, flawless job responsibilities, and standardized workflow at nucleic acid test sampling sites.
Acknowledgments
The authors thank all of the participants.
Funding: Jiangsu Province’s Health Commission Scientific Research Fund (No. S2017002) and Nanjing Medical Science and Technique Development Foundation (No. QRX17197).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jphe.amegroups.com/article/view/10.21037/jphe-21-100/coif). BZ serves as the unpaid Editor-in-Chief of Journal of Public Health and Emergency from January 2017 to December 2022. The authors report receiving funding from Jiangsu Province’s Health Commission Scientific Research Fund (No. S2017002) and Nanjing Medical Science and Technique Development Foundation (No. QRX17197). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Cite this article as: Lian X, Zhou X, Zhu B, Han L, Dou J, Zhao Y, Zhou L, Xu J, Liu J. Technical specifications for nucleic acid test sampling sites for prevention and control of COVID-19 pandemic in Jiangsu Province. J Public Health Emerg 2022;6:39.


