
Current strategies in chronic obstructive pulmonary disease management
Introduction
Chronic obstructive pulmonary disease (COPD) is a common condition characterized by persistent, progressive airflow obstruction. It is a result of an enhanced chronic inflammatory response to inhaled noxious particles or gases. COPD causes high airway resistance and over-compliant lung parenchyma, which leads to difficulty exhaling, air-trapping and hyperinflation. Chest mechanics become altered. The efficiency of the ventilation and gas exchange suffers. The end-result is often dyspnea and decreased exercise ability. The diagnosis of COPD requires evidence of obstruction (flow-limitation) on spirometry. The hallmark of COPD is a low, fixed ratio of the forced expiratory volume in one second (FEV1, the amount of air forcefully exhaled in one second) to the forced vital capacity (FVC, total amount of air exhaled during forced exhalation). By definition, that ratio has to be low, typically less than 70% (1).
Chronic bronchitis (chronic cough and sputum production) and emphysema (alveolar destruction) are often used synonymously with COPD. These terms reflect different aspects of the COPD disease state and describe common clinical and radiological phenotypes.
COPD contributes increasingly to the mortality rates across the globe and is currently the third leading cause of death (2). Continued tobacco abuse (increasing in middle income countries) and aging populations are the main factors responsible for this trend (1).
Various environmental exposures play a role in the development of COPD but, by far, smoking is the greatest risk factor. The World Health Organization (WHO) estimated about 5.4 million smoking related deaths in 2005, and they predict that that will increase to 8.3 million deaths per year by 2030. In low-income countries, indoor air pollution as a result of biomass fuel use and poor ventilation may be a risk factor as important as smoking (3). Other risk factors include occupational exposures, genetics, age and recurrent infections. The exact COPD prevalence is uncertain, as it is often underdiagnosed. Smoking rates in men peaked in the 1970’s in the US and are currently declining in many industrialized nations. Smoking rates in women, however, have lagged behind and in many underdeveloped countries are still on the rise (4). Deaths from COPD among women almost tripled between 1980 and 2000 in the US. The corresponding increase in men for the same period of time was only 13% (5). The incidence of COPD in women below 60 is much higher than in men—another alarming trend (6). In addition, women are physiologically more vulnerable to tobacco smoke (7). Because of the traditional gender roles in some societies, women’s exposure to biomass fuels tends to be higher (Figure 1).

The financial cost of COPD is difficult to estimate due to comorbid conditions and the high prevalence of undiagnosed cases. The national medical cost attributable to COPD in the USA alone in 2010 was estimated at $32.1 billion. This figure does not include lost productivity and is expected to increase to $49 billion by 2020 (8). The financial burden of COPD in the European Union is of similar magnitude (9). These estimates are likely conservative (10).
The goal of this article is to review the current strategies in COPD management and briefly outline potential future trends.
Objectives of COPD management
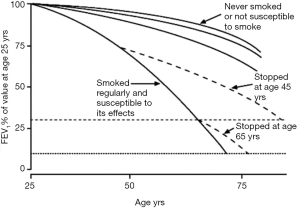
COPD is an irreversible, chronic condition. FEV1 begins to decline steadily early in life. Smoking accelerates this process. Smoking cessation returns the patient to their natural age expected rate of decline, but the existing damage is permanent (Figure 2). Early and accurate diagnosis is essential in order to slow down further deterioration. It is important to identify individuals with risk factors, especially smoking. Once the diagnosis is established, a strong emphasis is placed on risk factor elimination. Very few treatment options influence overall mortality once COPD has developed. The primary goals in the management of COPD are to slow progression and palliate symptoms. The evolution of the disease is monitored by spirometry. Preventing exacerbations and hospitalizations is important, both from the prospective of disease progression and quality of life. COPD trajectory is a step-wise curve and the lung function decline is often driven by exacerbations. Other important components of the chronic management of COPD are symptom control, early cancer detection, improved functionality and quality of life.
The most common symptoms in COPD are cough, with or without sputum production, and dyspnea. Wheezing may be a persistent or intermittent complaint. An exacerbation is defined as a worsening shortness of breath or cough with an increase in the sputum amount or a change in its color. It is estimated that, on average, a person with COPD will have two to three exacerbations a year. Older patients and smokers will have more. Exacerbations contribute significantly to the high cost burden of COPD and are taxing to the patient.
Lung cancer is more common in COPD than in the general population (12). This is not surprising with smoking being a common risk factor for both conditions. More importantly, the presence of COPD appears to be an independent risk factor for lung cancer even after controlling for smoking (13,14). Squamous cell carcinoma is the most common type in COPD patients (15). With rare exceptions, surgical resection is the only modality that can potentially provide cure. Thus, identifying early, potentially resectable lung neoplasms is an integral part of modern COPD management.
Unfortunately, even despite all efforts, many patients will develop end-stage COPD with resultant respiratory failure or incurable lung cancer. The clinicians should be prepared to focus on end-of-life care and to discuss their patients’ wishes pertinent to that. In particular, a discussion regarding mechanical ventilation is essential (16). It is a common misconception that palliative care can be administered only when restorative efforts have ceased. In fact, an early palliative multidisciplinary approach can improve survival in lung cancer patients (17).
Table 1 depicts a summary of the objectives of COPD management.
Table 1
| Identify and eliminate risk factors |
| Treat airflow obstruction |
| Slow disease progression |
| Decrease hospitalizations |
| Identify comorbid conditions (malignancy) |
| Improve functional status |
| Improve quality of life |
| Decrease exacerbations |
COPD, chronic obstructive pulmonary disease.
Non-pharmacological interventions
Elimination of environmental risk factors
By definition, COPD develops as an inflammatory response to inhaled noxious substances. Therefore, elimination of the exposure constitutes the most essential undertaking in COPD prevention. In particular, smoking cessation has repeatedly been shown to reduce morbidity and all-cause mortality (18). Smoking has been shown to accelerate the usual age related decline in FEV1. With cessation, the trajectory of the curve returns to the expected age related rate of decline (Figure 2).
Several nicotine replacement options are available to aid in quitting. Nicotine replacement therapy, in the form of long-acting nicotine patch and short acting gum or lozenges, has shown to improve cessation rate by as much as 50–70% (19). Bupropion—an oral antidepressant—increases the success rate at 1 year from 12% to 23% (20,21). More recently, varenicline—a partial agonist of the a4b2 neuronal nicotinic acetylcholine receptor—has replaced bupropion as a more effective option. Seventeen percent more people will achieve sustained abstinence when initially treated with varenicline compared to controls (22). Seizures, depression, suicidality and other neuropsychiatric reactions have been traditional concerns with both bupropion and varenicline. However, a recent large, double-blind study of more than 8,000 subjects failed to show a significant increase in moderate-to-severe neuropsychiatric adverse effects with either agent (23). The black box warnings have been lifted for both drugs by the regulators in the USA and similar steps have been taken in Europe.
Reducing biomass exposure is difficult in low-income areas due to the necessity for daily essentials (Figure 1). Education about ventilation and availability of cleaner energy sources can potentially decrease exposure (24).
There is inconclusive evidence over the utility of different forms of psychotherapy in smoking cessation. Hypnosis has shown anecdotal success, but a Cochrane review did not demonstrate any clear benefit (25). Cognitive behavioral therapy is a common adjunct therapy that improves cessation rate when combined with other pharmacological or non-pharmacological interventions, but there is little data about its use as a single modality.
Several other techniques have shown benefit as adjunct therapies. Quit lines have been beneficial in providing motivated patients with the resources available to them as well as emotional support throughout the process. They have proven effective when combined with other traditional tools (26). Likewise, dedicated cessation programs and clinics have shown to improve rates of smoking cessation (27).
Treatment of comorbid or mimicking conditions
Many conditions may present similarly to COPD, such as cardiac disease, bronchiectasis and psychiatric disease. Obtaining spirometry and a clear history is imperative to ensure the appropriate disease process is being treated.
Vaccinations
Preventing exacerbations and further respiratory complications is important in COPD. One of the ways to do so is through diligent administration of vaccines. Influenza is a common preventable cause of COPD exacerbation, morbidity and mortality. A yearly influenza vaccination reduces the incidence of influenza-related acute respiratory illness by 76% (28). In COPD patients, mortality is reduced by 40% on average during the influenza season (29). Mortality during an influenza epidemic is as much as 200 times higher in someone with combined pulmonary and cardiac disease (30). Administration of pneumococcal vaccination is an effective way to reduce the incidence of pneumococcal pneumonia. Its impact on mortality in COPD is less clear. The Centers for Disease Control (CDC) in the USA recommends a 13-valent pneumococcal conjugate vaccine (PCV) at age of 65 followed by a 23-valent pneumococcal polysaccharide vaccine (PSV) 1 year later. Early PSV administration can be considered in the subset of COPD patients under age 65 who continue to smoke. A booster of PSV should be administered at age of 65 or 5 years after the last administration (whichever is later) if the last received dose was prior to age of 65 (31).
Pulmonary rehabilitation
Severe dyspnea often limits COPD patients’ exercise ability. Pulmonary rehabilitation provides conditioning and breathing techniques to improve ventilation. Importantly, many programs also include patient education about the disease process and psychological support. There are many proven benefits of rehabilitation. Mortality benefit is still controversial, but rehabilitation has been shown consistently to improve quality of life and exercise capacity as well as to decrease dyspnea (32). It is also believed to decrease health care utilization by decreased number of hospital days and readmissions (33).
Oxygen therapy
Supplemental oxygen is one of the few treatments shown to provide a mortality benefit in selected COPD patients. A resting serum partial pressure of oxygen (pO2) ≤55 mmHg [oxyhemoglobin saturation (O2 sat) ≤88%] or pO2 between 56 and 59 mmHg with erythrocytosis or cor pulmonale indicate the need for supplemental oxygen. According to the NOTT trial, oxygen administered for at least 15 hours a day results in mortality reduction of 8.7% at one year and 18.4% at 2 years (34). Quality of life improves as well, but oxygen does not appear to have an impact on the rate of exacerbations or hospitalizations (35). There is no data to support oxygen therapy in purely nocturnal hypoxemia (36). A recent study found no benefit of oxygen therapy in moderate resting hypoxemia (O2 sat 89–93%) or exercise-induced desaturation (O2 sat 80–90%) (37).
Lung volume reduction surgery (LVRS)
LVRS involves resecting the apical parts of both lungs in selected patients with severe emphysema. It improves the elastic recoil and reduces hyperinflation. The respiratory muscle fibers return to a more favorable position on the length-tension curve resulting in improved ventilatory mechanics. Early attempts had unacceptable mortality rates. In 2003, the NETT trial showed a favorable outcome for those with severe, primarily upper lobe emphysema and poor post-rehabilitation exercise capacity. Quality of life was significantly improved. In addition, LVRS yielded a survival advantage in selected groups (38). More recently, new techniques have been in development that aim to achieve similar benefits using endoscopic procedures. Those techniques involve introducing a one-way airway valve, which results in a collapse of the hyper-inflated areas. While modest improvements in lung function, exercise tolerance and symptoms were noted, that was accomplished at the cost of more frequent exacerbations, pneumonia and hemoptysis (39).
Pharmacological treatments
Bronchodilators constitute the mainstay of the COPD drug therapy. These agents reduce the airway muscle tone, thus decreasing airway resistance and hyperinflation. Bronchodilation is most commonly accomplished via receptors on the surface of the airway smooth muscle cells.
β2-adrenergic agonists
Selective β2-agonists are derivatives of epinephrine/norepinephrine and have a predilection to bind directly to the β2-adrenergic receptors of the smooth muscle cells of the airways. Intracellular production of cyclic adenosine monophosphate (cAMP) is stimulated, which results in muscle relaxation. Common side effects include tachycardia, tremor, anxiety and insomnia. Their ability to cause more serious or even fatal tachyarrhythmias is less clear, but some studies have suggested so (40).
Inhaled short-acting β2-agonists (SABA), along with short-acting anticholinergics, are the mainstay of the management of acute symptoms of dyspnea and wheezing. With onset in minutes and duration of several hours, SABA are commonly used as a rescue inhaler for managing breakthrough symptoms. Their efficacy is greater when combined with short-acting anticholinergic agents (41). The frequency of as-needed administration of short acting bronchodilators can be used to grade long-term symptom control. Short acting inhaled bronchodilators can be administered by a metered dose inhaler (MDI) or a nebulizer (Figure 3). Oral formulations are available too but not recommended for routine use because of their less favorable side effect profile.
Long Acting β2-agonists (LABA), such as salmeterol and formoterol, are preferred to frequent short acting administration. Formulations are available that have onset within an hour and last 12–24 hours. LABA have been shown to improve lung function and quality of life while decreasing the rate of exacerbations and hospitalizations. A 13 mL/year (16 mL/year when combined with fluticasone) decrease in the rate of FEV1 decline was observed during a 3-year treatment with salmeterol compared to placebo. The clinical significance of this small improvement is uncertain (42). The benefits of LABA are additive when combined with inhaled corticosteroids (ICS). Unlike when used in bronchial asthma, monotherapy with a LABA does not cause increased mortality and is considered safe.
Anticholinergic (antimuscarinic) antagonists
Both short-acting (SAMA) and long-acting (LAMA) inhaled formulations are available. Anticholinergics work by blocking the effect of acetylcholine on the muscarinic cholinergic receptors of the airways, which reduces bronchoconstriction. The parasympathetic cholinergic activity is considered the dominant reversible component of airway obstruction in COPD (43).
Ipratropium is the most commonly used SAMA. It has an onset of 30–60 minutes with duration of action of 4–6 hours. This requires four times a day use if used around the clock. Its effect lasts longer than that of SABA (41). It is sometimes preferred over SABA as it appears to be less stimulating to the myocardium and, according to some studies, more effective (44-46). Due to the relatively lengthy onset of action, anticholinergic antagonists are not adequate as monotherapy for an exacerbation.
LAMA (tiotropium, aclidinium, umeclidinium) were developed to allow once or twice daily dosing with similar onset but with a duration of 12–24 hours. LAMA were shown to decrease symptoms and reduce exacerbations. Tiotropium significantly decreased the number of days to first exacerbation when compared to salmeterol (47) and improves quality of life (48). A small but significant (50–100 mL) improvement in FEV1 is seen, but the rate of FEV1 decline is unaffected (49). The benefits of LAMA are even greater when combined with LABA. The impact of LAMA on survival is less clear. A large randomized study demonstrated a survival benefit in moderate-to-severe COPD in subjects taking tiotropium versus controls, but mortality was a secondary outcome (50). A Cochrane review did not find convincing evidence of improved survival (48). Side effects of anticholinergics include dry mouth, nausea, constipation and urinary retention. Once thought to carry an increased risk of cardiovascular death and stroke (51), prospective studies have shown a good safety profile and even improved cardiac morbidity and mortality (50). Care should be exercised when anticholinergic agents are administered to patients with glaucoma or prone to acute urinary retention (e.g., prostate hypertrophy).
Corticosteroids
Airway inflammation is a prominent feature of COPD (52). Anti-inflammatory agents such as corticosteroids have been widely used as symptomatic therapy and in an attempt to slow disease progression.
ICS have been shown to improve quality of life and symptoms while decreasing the rate of exacerbations (53). They have little effect on the rate of decline of FEV1 (54,55). Retrospective pooled data have suggested a survival benefit, which may be even more pronounced when a LABA is added (56,57). The TORCH study, a large randomized trial, compared inhaled fluticasone and salmeterol in monotherapy and combination therapy. It did not show statistically significant mortality benefit after 3 years despite a promising trend. It did demonstrate, however, that both ICS and LABA slowed down modestly the FEV1 decline and improved overall health status. The combination therapy was more effective than either agent alone. ICS have been shown to have a better safety profile than systemic steroids, but increased incidence of pneumonia has been seen (53,58). A large cohort study suggested a decreased rate of lung cancer in large-dose ICS users (59), but this has not been confirmed by controlled trials. There is conflicting data over the incidence of osteoporosis. Oropharyngeal candidiasis and bruising are twice as common in ICS users compared to control subjects (54).
Systemic corticosteroids (parenteral or oral) are usually reserved for short-term use in exacerbations. As a maintenance treatment, they should be only used in patients who are not sufficiently controlled on preferred regimens. While effective in decreasing airway inflammation, the side effects such as elevated blood pressure, hyperglycemia, osteoporosis and neuropsychiatric manifestations among others limit their utility as a controller medication. Systemic steroids constitute the mainstay of treatment of exacerbations. Usually a short burst is used in conjunction with antibiotics and nebulized bronchodilators. A five-day course of daily 40 mg oral prednisone without a taper has proven to be as efficient as longer courses or higher doses (60).
Phosphodiesterase inhibitors
Methylxanthines (theophylline) are non-selective phosphodiesterase inhibitors. The inhibition of the phosphodiesterase enzyme raises the intracellular cAMP level in the smooth muscle cells resulting in bronchodilation. Theophylline is used primarily as an adjunctive agent. Beyond bronchodilation, it is believed to improve respiratory muscle function (61). Its use is limited by a very narrow therapeutic window and numerous drug interactions. Drug levels are affected by age and liver function. Side effects from elevated levels include arrhythmias, seizures and gastrointestinal upset. The use of theophylline may result in higher all-cause mortality in COPD patients (62). It is not recommended as a first line therapy.
Phosphodiesterase-4 inhibitors are more selective inhibitors that are believed to decrease inflammation without significant direct bronchodilation. Roflumilast is approved for COPD patients with frequent exacerbations (63). An improvement in FEV1 has been seen when added to ICS and LAMA therapy. The most common side effects include nausea, abdominal pain and weight loss due to anorexia.
Antibiotics
Macrolide antibiotics exert immunomodulatory and anti-inflammatory properties in addition to their antimicrobial effects (64). Azithromycin, when given daily to COPD patients prone to exacerbations, decreases the rate of exacerbations and improves quality of life. Hearing impairment and QTc prolongation can occur with prolonged use (65).
Mucolytics
Increased mucous secretion is common in many COPD patients. Mucolytic agents would appear to be useful additions to the COPD treatment. However, data supporting the routine use of mucolytics are lacking. N-acetylcysteine (NAC) has been the most studied mucolytic agent. It has no effect on the decline of lung function. Its use, however, can lead to a small but significant decrease in exacerbations. The benefits may be greater in individuals who have frequent exacerbations and hospitalizations (66). Mucolytics can be considered in patients with moderate or severe COPD not on ICS, especially during the winter season. There is no consistently agreed-upon dose.
Table 2 summarizes the most common treatment strategies in COPD today.
Table 2
| Therapy | Mortality | FEV1 decline | Rate of exacerbations | Symptoms/quality of life |
|---|---|---|---|---|
| Smoking cessation (18) | + | + | + | + |
| Pulmonary rehabilitation (32) | − | − | + | + |
| ICS (53,58,67) | − | +/− | + | + |
| LABA (53) | − | +/− | + | + |
| LAMA (48-50) | +/− | − | + | + |
| Theophylline | − | − | − | + |
| Roflumilast (63) | − | − | + | + |
| Azithromycin (65) | − | − | + | + |
| Mucolytics (66) | − | − | + | + |
| Oxygen (34-36) | + | − | − | + |
| LVRS (38) | + | − | − | + |
+, −, +/− indicate whether a benefit, no benefit or questionable benefit has been demonstrated in studies. COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists; LVRS, lung volume reduction surgery.
Novel management strategies
As our understanding of the molecular and cell biology of COPD continues to evolve, new management options are continuously explored. The challenges of these endeavors are several. COPD is a heterogeneous syndrome that may have disparate causes and pathomechanism (68). Efforts are required to identify phenotypes and individualize treatment. Creating a reliable and reproducible animal model has been difficult. None of the current models reproduces the exact changes seen in humans (69). The disease progression is a slow process, which requires long and expensive studies. Identifying reliable early markers and outcomes for assessing COPD progression and effectiveness of treatment would be a tremendous step in future research (70).
Newer members of the existing agent classes such as β-agonists, anticholinergics and inhaled steroids are continuously developed (71). At the same time, novel approaches are constantly sought. Prostaglandin pathways are emerging as a promising target. Affecting prostaglandin synthesis or signaling is considered by some a viable way to prevent vasoconstriction, inflammation and airway remodeling (72). Activation of matrix metalloproteinases has been implicated in the pathophysiology of COPD, especially in exacerbations (73). Inhibiting the activation or the synthesis of those enzymes may prove to be a feasible therapeutic target (74). Integrins are transmembrane proteins that play a role in various cell-to-cell and cell-to-matrix interactions. Their role and potential for therapeutic applications in airway inflammation and remodeling has been a target of recent research as well (75).
Anti-interleukin-5 injectable agents are effective therapy for difficult to control eosinophilic asthma. Trials are currently ongoing evaluating a potential role of these agents in COPD patients who demonstrate evidence of sputum eosinophilia (76).
There is an interest in developing new therapeutic agents to aid in smoking cessation. Blocking cannabinoid receptors in the central nervous system of rats has shown to reverse conditioned nicotine-seeking behavior (77). Human data were encouraging, but the development of the cannabinoid receptor blockers was discontinued because of side effects and a link to mental disorders (78,79).
Developing vaccines against drugs of abuse is an intriguing concept that has been investigated for years (80). Although attempts to invent a nicotine vaccine are currently limited to animal studies, the results are promising (81).
Summary
COPD is a common condition without a cure. Few modalities exist that provide a mortality benefit. The mainstays of treatment are aimed at relieving symptoms and preventing exacerbations. The main strategy in the COPD management is to identify early patients at risk and reduce risk factors, primarily smoking. Through continued research in epidemiology, cellular biology and pharmacology more treatment modalities are continuously being developed.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.02.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available from: http://goldcopd.org
- World Health Organization.The top 10 causes of death. Fact sheet N°310. 2013. Available online: http://www.who.int/mediacentre/factsheets/fs310/en
- Liu S, Zhou Y, Wang X, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax 2007;62:889-97. [Crossref] [PubMed]
- Mackay J, Amos A. Women and tobacco. Respirology 2003;8:123-30. [Crossref] [PubMed]
- Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance--United States, 1971-2000. MMWR Surveill Summ 2002;51:1-16. [PubMed]
- van Durme YM, Verhamme KM, Stijnen T, et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest 2009;135:368-77. [Crossref] [PubMed]
- Sørheim IC, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010;65:480-5. [Crossref] [PubMed]
- Ford ES, Murphy LB, Khavjou O, et al. Total and state-specific medical and absenteeism costs of COPD among adults aged >/= 18 years in the United States for 2010 and projections through 2020. Chest 2015;147:31-45. [Crossref] [PubMed]
- The economic burden of lung disease. European Lung White Book. Available online: http://www.erswhitebook.org/chapters/the-economic-burden-of-lung-disease/, accessed January, 2017.
- Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006;27:188-207. [Crossref] [PubMed]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645-8. [Crossref] [PubMed]
- Kiri VA, Soriano J, Visick G, et al. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J 2010;19:57-61. [Crossref] [PubMed]
- Koshiol J, Rotunno M, Consonni D, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One 2009;4:e7380 [Crossref] [PubMed]
- Turner MC, Chen Y, Krewski D, et al. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007;176:285-90. [Crossref] [PubMed]
- de Torres JP, Marin JM, Casanova C, et al. Lung cancer in patients with chronic obstructive pulmonary disease-- incidence and predicting factors. Am J Respir Crit Care Med 2011;184:913-9. [Crossref] [PubMed]
- Medarov BI. End stage pulmonary disease. Public Health Emerg 2016;1:2. [Crossref]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005;142:233-9. [Crossref] [PubMed]
- Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2012;11:CD000146 [PubMed]
- Ross S, Williams D. Bupropion: risks and benefits. Expert Opin Drug Saf 2005;4:995-1003. [Crossref] [PubMed]
- Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 1997;337:1195-202. [Crossref] [PubMed]
- Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 2015;313:687-94. [Crossref] [PubMed]
- Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507-20. [Crossref] [PubMed]
- Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg 2008;102:843-51. [Crossref] [PubMed]
- Abbot NC, Stead LF, White AR, et al. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev 2000;CD001008 [PubMed]
- Lichtenstein E, Zhu SH, Tedeschi GJ. Smoking cessation quitlines: an underrecognized intervention success story. Am Psychol 2010;65:252-61. [Crossref] [PubMed]
- Adelman WP, Duggan AK, Hauptman P, et al. Effectiveness of a high school smoking cessation program. Pediatrics 2001;107:E50 [Crossref] [PubMed]
- Wongsurakiat P, Maranetra KN, Wasi C, et al. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 2004;125:2011-20. [Crossref] [PubMed]
- Schembri S, Morant S, Winter JH, et al. Influenza but not pneumococcal vaccination protects against all-cause mortality in patients with COPD. Thorax 2009;64:567-72. [Crossref] [PubMed]
- Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med 1982;142:85-9. [Crossref] [PubMed]
- Kobayashi M, Bennett NM, Gierke R, et al. Intervals Between PCV13 and PPSV23 Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944-7. [Crossref] [PubMed]
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;CD003793 [PubMed]
- Man WD, Polkey MI, Donaldson N, et al. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004;329:1209. [Crossref] [PubMed]
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980;93:391-8. [Crossref] [PubMed]
- Stoller JK, Panos RJ, Krachman S, et al. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest 2010;138:179-87. [Crossref] [PubMed]
- Cranston JM, Crockett AJ, Moss JR, et al. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005;CD001744 [PubMed]
- Long-Term Oxygen Treatment Trial Research Group. A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med 2016;375:1617-27. [Crossref] [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest 2012;142:305-11. [Crossref] [PubMed]
- In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest 1994;105:1411-9. [Crossref] [PubMed]
- Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008;178:332-8. [Crossref] [PubMed]
- Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med 1984;311:421-5. [Crossref] [PubMed]
- Gross NJ. Ipratropium bromide. N Engl J Med 1988;319:486-94. [Crossref] [PubMed]
- Tashkin DP, Ashutosh K, Bleecker ER, et al. Comparison of the anticholinergic bronchodilator ipratropium bromide with metaproterenol in chronic obstructive pulmonary disease. A 90-day multi-center study. Am J Med 1986;81:81-90. [Crossref] [PubMed]
- Braun SR, McKenzie WN, Copeland C, et al. A comparison of the effect of ipratropium and albuterol in the treatment of chronic obstructive airway disease. Arch Intern Med 1989;149:544-7. [Crossref] [PubMed]
- Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093-103. [Crossref] [PubMed]
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014;CD009285 [PubMed]
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543-54. [Crossref] [PubMed]
- Celli B, Decramer M, Kesten S, et al. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:948-55. [Crossref] [PubMed]
- Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008;300:1439-50. [Crossref] [PubMed]
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645-53. [Crossref] [PubMed]
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89. [Crossref] [PubMed]
- Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med 2002;113:59-65. [Crossref] [PubMed]
- Sutherland ER, Allmers H, Ayas NT, et al. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax 2003;58:937-41. [Crossref] [PubMed]
- Sin DD, Wu L, Anderson JA, et al. Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax 2005;60:992-7. [Crossref] [PubMed]
- Soriano JB, Vestbo J, Pride NB, et al. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J 2002;20:819-25. [Crossref] [PubMed]
- Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008;300:2407-16. [Crossref] [PubMed]
- Parimon T, Chien JW, Bryson CL, et al. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:712-9. [Crossref] [PubMed]
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013;309:2223-31. [Crossref] [PubMed]
- Aubier M, Roussos C. Effect of theophylline on respiratory muscle function. Chest 1985;88:91S-97S. [Crossref] [PubMed]
- Horita N, Miyazawa N, Kojima R, et al. Chronic Use of Theophylline and Mortality in Chronic Obstructive Pulmonary Disease: A Meta-analysis. Arch Bronconeumol 2016;52:233-8. [PubMed]
- Martinez FJ, Calverley PM, Goehring UM, et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015;385:857-66. [Crossref] [PubMed]
- Martinez FJ, Curtis JL, Albert R. Role of macrolide therapy in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2008;3:331-50. [Crossref] [PubMed]
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689-98. [Crossref] [PubMed]
- Poole P, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2010;CD001287 [PubMed]
- Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;CD002991 [PubMed]
- Rennard SI. COPD heterogeneity: what this will mean in practice. Respir Care 2011;56:1181-7. [Crossref] [PubMed]
- Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2008;295:L1-15. [Crossref] [PubMed]
- Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J 2006;27:822-32. [Crossref] [PubMed]
- Devillier P, Garrigue E, D'Auzers G, et al. V0162 a new long-acting bronchodilator for treatment of chronic obstructive lung diseases: preclinical and clinical results. Respir Res 2015;16:68. [Crossref] [PubMed]
- Zaslona Z, Peters-Golden M. Prostanoids in Asthma and COPD: Actions, Dysregulation, and Therapeutic Opportunities. Chest 2015;148:1300-6. [Crossref] [PubMed]
- Papakonstantinou E, Karakiulakis G, Batzios S, et al. Acute exacerbations of COPD are associated with significant activation of matrix metalloproteinase 9 irrespectively of airway obstruction, emphysema and infection. Respir Res 2015;16:78. [Crossref] [PubMed]
- Daheshia M. Therapeutic inhibition of matrix metalloproteinases for the treatment of chronic obstructive pulmonary disease (COPD). Curr Med Res Opin 2005;21:587-94. [Crossref] [PubMed]
- Wright DB, Meurs H, Dekkers BG. Integrins: therapeutic targets in airway hyperresponsiveness and remodelling? Trends Pharmacol Sci 2014;35:567-74. [Crossref] [PubMed]
- Efficacy and Safety of Mepolizumab as an Add-on Treatment in Chronic Obstructive Pulmonary Disease (COPD). Available online: clinicaltrials.gov/ct2/show/NCT02105961, accessed January, 2017.
- Cohen C, Perrault G, Griebel G, et al. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 2005;30:145-55. [Crossref] [PubMed]
- Steinberg MB, Foulds J. Rimonabant for treating tobacco dependence. Vasc Health Risk Manag 2007;3:307-11. [PubMed]
- Cahill K, Ussher MH. Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database Syst Rev 2011;CD005353 [PubMed]
- Kantak KM. Vaccines against drugs of abuse: a viable treatment option? Drugs 2003;63:341-52. [Crossref] [PubMed]
- Jacob NT, Lockner JW, Schlosburg JE, et al. Investigations of Enantiopure Nicotine Haptens Using an Adjuvanting Carrier in Anti-Nicotine Vaccine Development. J Med Chem 2016;59:2523-9. [Crossref] [PubMed]
Cite this article as: Keen C, Medarov BI. Current strategies in chronic obstructive pulmonary disease management. J Public Health Emerg 2017;1:26.


