
HIV metabolic clinic: can the journey start from a UK clinic to a sub-Saharan African nation?
Introduction
For some years now, the statistics for morbidity and mortality from infectious diseases involving especially HIV have drastically declined (1). This can largely be attributed to the better understanding of the disease process, which in turn has led to more effective treatments namely in the form of cART (2). Consequently, non-communicable diseases such as MetS, fatty liver, HIV-associated nephropathy (HIVAN), CVD and endocrine disorders have become a major public health concerns worldwide. This has posed a great challenge to global health planners due to its implications in multiple lifestyle disease and concerted efforts around prevention. Considering the world of affluence and sedentary lifestyles, increasing obesity and increasing tastes for ‘unhealthy foods’, all indicate that the challenges posed by MetS will worsen if concerted actions are not undertaken (1). In HIV naive individuals, National Health and Nutrition Examination Survey (NHANES) data 2003–2012 highlighted a prevalence rate of 18.3% among those between 20–39 years while it increased to 46.7% among those aged 60 years or older with higher incidence occurring in women. On the other hand, in HIV infected individuals, although the numbers are still debatable, prevalence for MetS ranges from 11.2–45.4% (1,2).
Studies over two decades ago, have persistently linked HIV infection to MetS and the likely hypothesis shows that chronic low-grade inflammation seen in HIV is responsible and further exacerbated by the development of obesity in susceptible individuals (1,3). This is now further compounded by using the disease specific medication cART that can further exacerbate some of the initial changes found to occur in the early stages of HIV infection which involve further components. A case in point being, protease inhibitors (PIs) (a major cART drug) causes selective inhibition of glucose transport in adipocytes1. Nevertheless, the risk of not being on cART is overwhelmingly greater than the resultant metabolic adverse events (4).
To combat this, a weekly metabolic clinic was set up in MKUH. Although the establishment of this clinic is still fairly new and constantly evolving to meet the specific needs of our patients, it has to date met considerable demands by service users and hence has great potential of becoming popular and of huge future benefits. Since SSA is the major hot spot for individuals living with HIV, it would be a very useful system to be adopted. It would go a long way in the management of complex cases resulting in huge cost saving benefit and efficient use of healthcare resources in SSA HIV specialists and the metabolic team work effectively together in order to exchange and deliver evidence base knowledge to prevent severe complications associated with long term non-communicable diseases seen in HIV patients (5).
The aim of this paper is to see why metabolic clinics for HIV patients is required in Africa by looking at the prevalence, complications, mortality rates and cost implications in Africa of HIV associated diseases such as MetS, DM, dyslipidemia, CVD, NAFLD, HIVAN and endocrine disorders.
Methods
Identification of relevant studies
We reviewed the published literature on the major publication databases from 1996 to current dates. We limited our search from 1996 due to the fact that cART was introduced from this year and in 1998 the first MetS criteria was defined according to World Health Organisation (WHO) guidelines (6,7). The comprehensive electronic search included standard medical databases such as PubMed, Medline and Google Scholar. In the search we used not only the combination of Medical Education and Stimulation Hubs (MESH) terms, free words involving prevalence of MetS and HIV in SSA countries but also the following terms: dyslipidemia, metabolic syndrome (MetS), cardiovascular risk and HIV, NAFLD, HIVAN, endocrine, thyroid, adrenal, calcium, metabolic clinic in Africa and ART regimen. Moreover, citations were traced in certain identified articles by scanning the reference lists of the already selected review papers.
Seven specific HIV-associated chronic non-communicable diseases were reviewed which included MetS, DM, dyslipidemia, CVD, NAFLD, HIVAN and endocrine disorders.
Selection of included studies
We independently reviewed the studies where relevant using abstract and full text for inclusion. Included studies had to be African population based, inclusive of adults diagnosed with HIV on CART, reports of prevalence and management of HIV associated chronic conditions by different subgroups published in English language-based peer reviewed journals. No restrictions were made on sample size or methods or study setting and if multiple surveys were reported in one article, each survey was mentioned and counted separately if relevant. The sections that follow report the findings of the selected publications.
Why Metabolic Clinic for HIV patients is needed in SSA
Due to high prevalence of diabetes, hypertension, NAFLD, HIVAN and endocrine disorders with HIV. In the following sections we will explain about the high prevalence of these metabolic disorders and how HIV metabolic can help with regard to these issues.
MetS among HIV patients in Africa
MetS is classified as a group of complex interrelated risk factors for CVD and DM. Factors which include dysglycaemia, raised blood pressure, elevated triglyceride levels, low high density lipoprotein cholesterol levels and obesity (particularly central adiposity) (5,8). Since the introduction and widespread use of cART from the mid-1990s in HIV infected individuals there has been a dramatic decline in AIDS and immunodeficiency-related events and individuals infected with HIV now have increased life expectancies (4). As a result, individuals are not only exposed to the long-term effects of administering cART and the condition (which has been proven to have an independent effect on cardiovascular risk), but also to the effects of ageing seen amongst the general population for instance DM and CVD (8). It has however been demonstrated clearly that MetS is common and there is a rising prevalence worldwide. Studies have shown that the prevalence ranges from 11.2–45.4% depending on the sampled population and criteria used. Despite the disparities, patients on cART were identified to have a higher prevalence of MetS compared to cART naive counterparts (2).
Complications of MetS include cardiomyocyte toxicity and endothelial cell apoptosis leading to endothelial dysfunction and vascular damage with an increase in resting energy expenditure, fat oxidation and the increase in food intake (1). As the most heavily impacted countries with individuals infected with HIV are particularly seen in SSA, establishing a metabolic clinic here would provide significant benefits. Similar structure currently being practiced in MKUH in the UK could be adopted. This clinic has a 30 minutes slot for each patient where extensive MetS risk factors and lifestyle issues can be discussed within the normal HIV clinic where patients would not only see their usual HIV specialist but that of a metabolic medicine specialist and a dietician (5).
Diabetes mellitus (DM) among HIV patients in Africa
DM is a chronic disease with an increasing global burden. It is estimated that by the year 2030, 439 million adults will be diagnosed with diabetes, and developing countries will see a 69% increase in newly diagnosed adults (9). On the Global Health Observatory data repository which is part of the WHO; it estimates the global HIV population of all ages to be around 36.9 million, with the highest population region being in SSA with a total of 25.7 million infected individuals (10). Thus, highlighting the great global burden faced within the healthcare setting due to these two chronic diseases. The treatment and management of people with HIV has greatly improved over time, and the introduction of cART has helped reduce the rate of progression and prevalence of the disease. However, this has then led to an increase in chronic complications and co-morbidities for instance the occurrence of DM (11). It was found that HIV patients on cART experienced a number of metabolic complications of which included insulin resistance due to disturbances in the molecular pathways in the metabolism of glucose and insulin (12).
There have been a few studies carried out in SSA looking at individuals living with chronic diseases such as HIV, though the data reported with regards to the effects of chronic disease on HIV infected individuals is low (13). However, these studies identified that African patients with HIV had a poor control of blood glucose. Prevalence nonetheless, varied depending on the country and the type of data collected. In some African countries the estimated prevalence of HIV patients with diabetes varied from 2.1% to 26.5% and the estimated prevalence of HIV patients with glucose intolerance varied from 20.2% to 43.5% (14).
Another study found that the prevalence of DM amid people living with HIV varied from 1% to 26% and the prevalence of pre-diabetes in this population group was 19% to 47%. To summarise, these studies looked at the prevalence of DM amongst people with HIV in 8 SSA countries which included Nigeria 2.3%, Cameroon 2% to 26%, Ethiopia 6.4%, Kenya 1.5%, Tanzania 0.7% to 18%, Rwanda 0.5%, Zimbabwe 08% to 2.1% and South Africa 2.2% to 17% (15).
Diabetic complications can be either macrovascular or microvascular. In a study performed on diabetic Malawian HIV-naive patients, it was found that 7.3% were reported to have had a stroke and 6.7% reported foot ulceration. These results were similar to other studies which were conducted in other parts of Africa. The study also looked at microvascular complications which were considerably more in comparison to the macrovascular complications; 34.7% were reported to having nephropathy, 34.7% having retinopathy and 46.4% reported to having neuropathy. In this study they also looked at diabetic patients with HIV and found the only abnormal reading was the albuminuria. It is known that HIV can affect the kidney and cause the development of HIVAN which can present with proteinuria. It is also noted that, peripheral neuropathy is common in both DM and HIV. In another study carried out in Malawi it was found that patients with only a diagnosis of HIV also experienced peripheral neuropathy, therefore in patients with HIV and diabetes who have neuropathy it may be difficult to distinguish whether the neuropathy was caused by DM or HIV (16). The prevalence of renal disease in patients with HIV and diabetes in Africa has not been well documented.
Dyslipidemia among HIV patients in Africa
Most studies have found that HIV patients on combination cART, dyslipidemia is common and manifests as high total cholesterol, high low-density lipoprotein count (LDL-c) and high triglycerides (TG) as well as low high-density lipoprotein count (HDL-c) (14,17,18). These are said to correlate positively with the level of CD4+ cell counts (17). Acute phase responses are caused by chronic infections accompanied by decrease in lipoprotein lipase activity and subsequently increase in TG, which promote conversion to HDL and lipolysis thus, low HDL-c (19,20). In developed countries, risk assessments for CVD is essential as such it could be said that HIV patients living in Africa would benefit from lipid specialist to reduce CVD. According to a paper by Ntobeko Ntusi [2018], South Africa has one of the largest populations worldwide of HIV patients receiving cART as well as having a significant number of these patients being treated with dyslipidaemia. Dave et al. [2016] reports an 85% prevalence of dyslipidemia in cART treated HIV patients and 90% in those on ART-naïve (17). Hypercholesterolaemia was found in 32.3% of patient living with HIV (PLWHIV), high LDL-c in 9.5% and low HDL-c in 45.7% (14,17). In other African countries higher prevalence of dyslipidaemia was reported; Kenya for example reported a 63.1% associated with high TG, LDL-c and total cholesterol. In Tanzania, high TG was prevalent in 28% and low HDL-c in 67%, which was associated with low CD4+ counts (P<0.001) (10). In Nigeria high TG, LDL-c and cholesterol were seen at 35%, 24% and 28% respectively (21,22). Prevalence of dyslipidemia was reported as 70.2%, 31% and 56.9% in Cameroon, Malawi and Ethiopia respectively (23-25).
Additionally, dyslipidaemia can be precipitated by certain HIV treatments such as protease inhibitors (PIs), nucleoside reverse transcriptase inhibitors (NRTIs) and non-NRTIs (5). Virological and immunological status is improved dramatically with PIs containing cART treatment however, it is said to heighten HIV related dyslipidaemia in the form of increased TG, LDL-c, decreased HDL-c and buildup of Apo-C III and Apo-E (5,26,27). Chen et al. [2002], states that the prevalence of dyslipidaemia in PLWHIV on cART therapy is around 70–80% and this is correlated with the use of all accessible PIs (18). Several studies have reported incidence of coronary heart disease in PLWHIV on cART treatment including PIs of at least a threefold increase when compared to the general population; as well as acute pancreatitis in patients with severe increased TG associated with PI therapy (26,28-30). Other treatment options such as NRTIs also show correlation with high TGs and a few have also been associated with a 26% increase independent risk in frequency of myocardial infarction (MI) per year of cART exposure (P<0.001) but the DAD cohort study [2008] also states that dyslipidaemia contributed only partly to the rates of MI being increased (31).
It should be noted that the pathogenesis showing the link between dyslipidaemia and immune dysfunction remains a mystery (32). However, two different studies, one in the USA and one in Nigeria both showed a strong link between levels of serum LDL and CD4+ cell counts. They observed that as CD4+ cell count increased so did the prevalence of dyslipidemia (33,34). Furthermore, an increase in comorbidities such as dyslipidaemia, hypertension and CVD was noted due to the fact that PLHIV live longer (35). In SSA countries, CVD has become one of the major causes of death in HIV patients due to the high prevalence of DM, dyslipidemia and MetS (14). Dyslipidaemia is thus said to be largely associated with CD4+ cell count and the use of ART most especially PIs.
Cardiovascular disease (CVD) among HIV patients in Africa
It is well noted that people with HIV now live longer but experience many metabolic complications and therefore this population group are at an increased risk for CVD. Moreover, it has been noted that in African countries a significant cause of death in HIV patients is due to CVD (14). In a study conducted in Ethiopia, it was found that the number of deaths due to HIV and its associated immunodeficiency effects had dropped by 70% since 2005 due to the use of cART. Yet, it was still considered one of the five leading causes of death in Ethiopia in 2015 because of the cardiovascular risk the use of cART poses (36).
During the early stages of the HIV pandemic, cardiac diseases in HIV infection were noticed and documented (37). After the introduction of cART HIV became a chronic disease with further increased risk of CVD (38). There is also a difference seen in the nature of CVD between the pre-cART and post cART eras. In developed countries during the post-cART era cardiac diseases such as coronary artery syndrome, lipodystrophy and MetS were commonly seen in HIV infected patients. Whereas, in developing countries such as SSA, conditions such as cardiomyopathy, pericarditis and pulmonary hypertension are more common, which in developed countries was more prevalent among HIV patients in the pre-cART era and is likely due to the healthcare infrastructure picking these co-morbidities up in developed nation settings.
In the Heart of Soweto, the largest contemporary study involving 5,328 patients linking cardiac diseases to HIV in SSA found that 10% of the patients with CVD were HIV-infected. Within these, 37.8% had HIV-related cardiomyopathy, 12.5% had pericarditis/pericardial effusion, 11.2% had valve disease, 7.1% had hypertension, 6.9% had other heart disease, 6.6% had right heart failure, 6.2% had hypertensive heart failure and 2.7% had coronary artery disease. Hypertension and obesity (which are major risk factors for developing CVDs) were also largely prevalent among HIV infected patients in countries such as Kenya (39). A study involving 79 patients in the National Hospital of Ouagadougou (Burkino Faso), showed the following prevalence of HIV-associated CVD: 57% had cardiomyopathy, 32% had pericarditis, 5% had pulmonary hypertension and 2 cases had MI (40).
Most of the types of HIV-associated cardiac conditions were linked to increased mortalities. In developing countries, it was seen that HIV-associated cardiomyopathy resulted in high mortality rates due to congestive heart failure. Also, pericardial effusions in patients with advanced HIV disease increased the risk of mortality by 2.2-fold (41). In Sub-Saharan Africa, even with Cart-therapy, HIV-associated tuberculosis pericarditis had a high mortality risk between 20% to 40%, which has resulted in an increased need for treatment for this possibly curable disease in HIV patients. A study in Burkino Faso, also reported that mortality rate was 15% in patients with cardiovascular complications in PLWHIV.
As explained in the section above most of the research and literature available in SSA is focused on infectious and inflammatory causes of CVD. But as we can see there is a growing population of degenerative cardiac conditions among HIV patients in Africa due to different factors such as increased and extended use of cART, ageing population and urbanisation. A Kenyan study showed that the least amount of the budget (for cumulative spending for global health and overall disease burden) was allocated for non-communicable diseases even though they result in the highest number of disability adjusted life-years and overall mortality (39). Though there is not a lot of data provided regarding the financial burden associated with HIV infected individuals and their increased risk to DM and cardiovascular conditions, a study conducted within the Democratic Republic of Congo found a number of issues faced by patients. Some of these issues included the financial inability to travel to get to the clinics, pay for treatments and following a recommended diet which further impact PLWHIV management of their condition (42). The use of cART has helped improve the lives of the vast majority of PLWHIV, but it has also caused a number of complications which need to be effectively managed which has led to a holistic management of care.
There is a need for expansion of the HIV programs to also address the significant overlap between non-communicable diseases (such as cardiovascular condition) and communicable diseases. Metabolic clinics for PLWHIV is a possible way to monitor and treat these long-term cardiovascular complications before it progresses and causes further rise in morbidity and mortality. The clinics can also help identify and manage cardiovascular risk factors such as hypertension, dyslipidemia and obesity.
Nonalcoholic fatty liver disease (NAFLD) among PLWHIV in SSA
In PLWHIV, NAFLD has become increasingly documented although not well characterised. Emerging evidence suggest that excess use of cART drugs together with precursor factors such as insulin resistance and dyslipidaemia; could be important contributors to NASH and consequently liver cirrhosis (43). Notably, HIV patients with Hepatitis C virus (HCV) can also have NAFLD (44,45); however abnormal liver enzymes are said to occur in 40–60% of patients currently on cART regardless of the presence of HCV or Hepatitis B virus (HBV) which is far greater than 8% seen in general population (46).
Prevalence of NAFLD in a population-based study was found to be 26% whilst associated with an increase in insulin resistance and γ-glutamyl transpeptidase (46). Sterling et al. [2014], mentions a large study of nearly 6,000 HIV patients showing in subjects without HCV, the prevalence of abnormal AST and ALT was 75% and 55% respectively. Due to the lacking evidence of HIV patients with increased liver enzymes without viral hepatitis, they hypothesise that this increase in enzymes are due to steato-hepatitis as a result of hepatotoxicity of cART (46). Vernon et al., emphasises even further the high prevalence of NAFLD in PLWHIV treated with cART (47). In a cohort study done in Nigeria, fatty liver was detected in 13.3% of patients (113 patients) with HIV/AIDS on long term ART (21). Olufunmilayo et al. [2009] mentioned that the high prevalence of NAFLD may be due to liver granulomatous reaction as a result of viral infection and a host of other opportunistic infections (21). Although NAFLD is still not well characterised in literature as explained above, it certainly can benefit from not only metabolic clinics looking at the effects of excess use of long-term cART drugs and monitoring liver functions for any abnormal enzymatic results but also more research to identify the possible risk factors and complications in PLWHIV together with or without HCV.
HIVAN in SSA
HIVAN is commonly seen in African PLWHIV and often results in end stage renal failure (48). In this age of prolonged survival of PLWHIV and cART use, there is a great potential for an epidemic of HIVAN (seen almost exclusively in people of African descent) among the 30 million HIV patients in SSA (49). Early screening for HIVAN can be done by testing for microalbuminuria. Then those with persistent microalbuminuria can undergo renal biopsy to confirm HIVAN. This will help detect HIVAN at an early stage, which in turn can help in early treatment and prevent the disease from progressing. In most SSA countries due to resource limitations and high levels of PLWHIV, early detection of acute and chronic renal disease is delayed and preventative treatment is not started on time.
The prevalence of HIVAN varies greatly in different countries around the world extending from 4.7% to 38% (50). There are not many studies to determine the prevalence of HIVAN in SSA as a whole, although there are some studies looking at the prevalence in specific African countries such as South Africa (6%), Kenya (25%), Nigeria (38%), Ivory Coast (26%), Uganda (20–48.5%), Tanzania (28%), Zambia (33.5%) (51). It was also found that patients with HIVAN progressed to end stage kidney failure within a few months post-diagnosis (52). Data suggests that 3.5–12% (1–3.5 million) of HIV infected Africans are at risk of developing HIVAN which means SSA is at the brink of facing an epidemic of renal failure, which it is unprepared for. HIVAN is the predominant underlying pathology in 20% of HIV patients that present with acute renal failure. Twenty percent of those with HIVAN also have additional acute abnormalities. Data from Groote Schuur Hospital in Cape Town showed that acute renal failure in HIV patients can be caused by acute tubular necrosis (which is defined as acute kidney injury due to sepsis, nephrotoxic drugs or hypovolemic shock), malignant hypertension, drug related acute eosinophilic interstitial nephropathy or renal tuberculosis (53). It also showed that causes of chronic renal failure in HIV patients other than HIVAN include HIV immune complex disease, membranous glomerulonephritis, mesangiocapillary glomerulonephritis, mesangial proliferative glomerulonephritis, idiopathic focal segmental glomerulosclerosis, hypertensive nephrosclerosis and diabetic nephropathy.
A study in UK involving 20,132 patients showed that reduced baseline kidney function in HIV patients result in increased mortality and progression of renal disease (54). With lower estimated glomerular filtration rate (eGFR) in these patients, the risk of death increased. An eGFR of <30 mL/minute in HIV patients showed a 3-fold increase risk in mortality. The study also found that there was a statistically significant (P<0.001) interaction between eGFR and ethnicity and that there was an evident increased risk of mortality with decreasing eGFR in black patients. Another study done in Zambia showed that, there was increased mortality risk at or before 90 days of initiation of ART in HIV patients with renal insufficiency (55). This also showed that worsening renal insufficiency was associated with increased mortality.
The African continent consists of many countries that are poverty stricken and this impacts on the healthcare provided and the clinical decisions made (53). WHO statistics in 2012 for African countries show that the gross domestic product per capita for 41 out of 45 African countries is less than $8,000 with the lowest being $246. This shows that most countries cannot meet the average annual cost of renal replacement treatment per patient which was estimated to be $9,130 for hemodialysis and $8,319 for continuous ambulatory peritoneal dialysis in South Africa in 2004 (53). Therefore, advanced renal disease due to HIV essentially means a death sentence in most African countries. Diagnosing HIV-related nephropathy, monitoring and treating it before it progresses into advanced renal disease that require renal replacement therapy can therefore help reduce the cost implications on the country as well as reducing the risk of death among patients who cannot afford the treatment.
Metabolic clinics for HIV patients in Africa can help monitor renal function regularly and provide appropriate treatment at an early stage to control it before it progresses, as we are going towards a time where renal failure is possibly going to be rampant among HIV patients.
Endocrine disorders among HIV patients in SSA
It is known that HIV can cause functional abnormalities in endocrine systems and their individual organs. However, in the last two decades the introduction of cART has given rise to the increased rates of endocrine pathologies in individuals infected with HIV. The relationship between the endocrine system and HIV is a rather intricate one and this aspect of medicine is gradually developing (56). The endocrine system overseas the control of a number of systemic functions, however in this review we will be focusing specifically on calcium, thyroid and adrenal gland functions. In HIV infection the adrenal gland is the most likely gland to be affected in the endocrine system, and therefore highlighting the importance of identifying complications such as adrenal insufficiency. The prevalence of adrenal insufficiency is quoted around 0.5% to 72% highlighting the discrepancy in data. It is also understood that a potential cause of adrenal insufficiency in HIV patients is due to cytomegalovirus (CMV) infection. However, there are other opportunistic infections such as cryptococcosis, mycobacterium avium intracellulare, toxoplasmosis, pneumocystis carinii, histoplasmosis and blastomycosis.
In a cross-sectional study involving 66 patients from South Africa the prevalence of hypoadrenalism was found to be 27% and the contributory infections mentioned above were present in the test population. It was found that 100% of the cohort were infected with CMV and 68.2% were found to be infected with tuberculosis (57). In a study assessing adrenal insufficiency conducted on 43 Nigerian individuals infected with HIV it was found that the prevalence in this study was 34.8%, however these results of adrenal insufficiency were obtained via a reduced cut-off value and therefore could have potentially underestimated the actual proportion of individuals with adrenal insufficiency. The individuals in this study were also treatment naive and therefore no comment can be made on the impact of antiretrovirals in the management of HIV and adrenal insufficiency within this study (58). In a study focusing on the Senegalese population infected with HIV on antiretroviral therapy (ART) it was found that a common complication of these individuals is reduced BMI in comparison to the control group which was not infected with HIV. It was found in the infected group that they had reduced quantitative ultrasound (QUS) bone mineral density which may potentially be associated with their lower BMI, however no correlation was found between ART and reduced QUS bone mineral density (59).
In a study looking at the effects of thyroid dysfunction on reproductive hormones in premenopausal women in Nigeria, it found that in symptomatic individuals infected with HIV there was no difference between FSH, LH, progesterone and oestradiol in the follicular and literal phases of the menstrual cycle. However, in the healthy group there were differences in hormones in the two phases. Therefore, the lack of physiological normality seen in HIV patients may lead to reproductive dysfunction. It is found that lowered levels of progesterone in the luteal phase may affect pregnancy and potentially cause a spontaneous abortion, and even further reductions of these hormones mentioned above could cause menstrual problem such as failure of menstruation to occur (60).
In a study in Uganda looking at functional adrenal insufficiency (FAI) in critically ill HIV patients who presented to the medical emergency unit, also briefly discussed the mortality rates within this study. The study included 113 patients in total but only 21 of the patients were diagnosed with FAI. In the study there was no significant difference of mortality rates between each group. The group with FAI had a mortality rate of 19% which was 4 patients and the other group with normal adrenal function had a mortality rate of 33% which was 30 patients. The mean cortisol level of the people who died was higher in comparison to the patients whom were discharged, therefore suggesting that patients with a higher cortisol level may have a poorer prognosis. However, of the patients who died in the FAI group had either a diagnosis of cryptococcal meningitis with intra-cerebral edema or respiratory complications as a result of pulmonary Kaposi’s sarcoma.
However, with such a small sample size of 21 patients with FAI it’s is very difficult to conclusive identify the causal reason for mortality. It was found that patients with FAI who received corticosteroids all survived whereas patients with FAI who didn’t receive corticosteroids, 30% of these individuals died in hospital, highlighting a potential significance in corticosteroid treatment for FAI, which needs to be researched and understood (61).
Very little resources are available discussing the mortality rates of patients with HIV and endocrine disorders, therefore highlighting the importance to carry out further research to fully understand the significance and rates of mortality within this population group.
A paper looked at the cost of care of 469 individuals infected with HIV in an inpatient setting in South Africa. In the study there was only one admission of an HIV infected individual with an endocrine and metabolic pathology and the cost of the admission using United States dollars (USD) was 2,472. For this case the majority of the cost was accumulated from medical staff which was 1,063 USD and fixed costs which included space, equipment and hotel costs which totaled to 905 USD (62).
More research needs to be carried out on the pathology of endocrine disorders and how they were impacted by HIV and their respective treatment options. In addition, more needs to be understood about the long-term complications and mortality risk of endocrine disorders within this population group in the African continent. All of these will help ensure endocrine pathologies are quickly picked up and managed appropriately.
Potential benefits of a metabolic clinic dedicated for HIV care
Aforementioned, HIV which was initially considered as an invariably fatal disease especially in developing countries; however, it has now become a chronic condition primarily due to advances in therapeutics. Now HIV patients have a lower chance of succumbing to opportunistic infections but at the expense of developing MetS and other chronic conditions (1). It is thus essential and beneficial to establish a clinic fully equipped with the expertise to combat this. Having dedicated metabolic clinics in place will not only ensure HIV patients worldwide have a better quality of life but also serve as a preventative measure to the likely complications associated with MetS and other long-term conditions (4,5). SSA African countries will in the long term save money and health resources and as a consequence heavily reduce these burdens on their healthcare systems. The financial savings here could be channelled to other deprived healthcare areas, thus creating a more cost effective and efficient use of resources and funds available (2). Likewise, since the clinic will likely to provided be within the same HIV services patients will be more likely to attend or comply to advise given as they will feel more comfortable with the same healthcare professionals who care for their HIV management in a seamless and confidential manner. A guideline for patients with multi-morbidity as well as a metabolic database should be established and made available to help with the purposes of research, auditing and teaching. Having this in place will mean that the patients get a multidisciplinary decision each time they attend clinic not only with regards to their risk associated with chronic non-communicable diseases but also with managing their HIV and medications as this clinic will create a platform for all specialist involved to communicate effectively (5). For further possible potential benefits of metabolic clinic please see Figure 1.
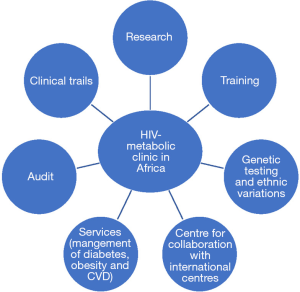
Conclusions
It is shown through much accumulated data that HIV patients have a longer life span due to the medications available and as such are also at risk of specific age-related disorders seen in the general population. Not forgetting that these medications have also shown to increase the likelihood of MetS such as DM, obesity, dyslipidemia, HIVAN, NAFLD and increased risk of CVD in these patients. In African countries a major cause of death in HIV patients is CVD, which is said to be due to increased prevalence of MetS. A small but significant proportion of morbidity and mortality among HIV patients in Africa will be due to CVD, especially because of the large HIV population in SSA that also suffers from high mortality rates due to other reasons. Risk reduction and lifestyle changes play a vital role in the management of these patients and thus introducing a metabolic clinic will not only be able to meet the demand for clinical services, similarly it will ensure cautious selection of cART drugs in response to the cardiovascular risk factors that patients present with. In addition, it will provide further ground for data collection and research in the future (Figure 1).
Acknowledgments
Dr. Mital and Dr. Ahmed would like to thank all the staff in the department of HIV and Blood Borne Viruses, Milton Keynes University Hospital, NHS Foundation Trust, Milton Keynes, UK for their support for HIV metabolic clinic.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2018.09.02). MHA serves as an unpaid editorial board member of Journal of Public Health and Emergency from August 2017 to July 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abhijit S. A Metabolic Syndrome and HIV infection. J HIV Retro 2016;2:1.
- Obirikorang C, Quaye L, Osei-Yeboah J, et al. Prevelance of Metabolic syndrome among HIV infected patients in Ghana: A cross sectional study. Niger Med J 2016;57:86-90. [Crossref] [PubMed]
- Brian R, Andrew B, James H. Strengthening Routine data systems to track the HIV epidemic and guide the response in sub saharan Africa. JMIR Public health Surveil 2018;4:e36.
- Paula AA, Falcao MC, Pacheo AG. Metabolic syndrome in HIV-infected individuals:underlying mechanisms and epidemiological aspects. AIDS Res Ther 2013;10:32. [Crossref] [PubMed]
- Ahmed MH, Woodward C, Mital D. Metabolic clinic for individuals with HIV/AIDS: a commitment and vision to the future of HIV services. Cardiovasc Endocrinol 2017;6:109-12. [Crossref]
- New therapies: new hope. Reports from the International Nursing Satellite symposium and the Eleventh International Conference on AIDS, Vancouver, Canada, 6-12 July, 1996. Midwifery 1996;12:205-6. [Crossref] [PubMed]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53. [Crossref] [PubMed]
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640-5. [Crossref] [PubMed]
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4-14. [Crossref] [PubMed]
- GHO | By category | Number of people (all ages) living with HIV - Estimates by WHO region. Available online: http://apps.who.int/gho/data/view.main.22100WHO?lang=en
- Kalra S, Kalra B, Agrawal N, et al. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr 2011;3:2. [Crossref] [PubMed]
- Samaras K, Wand H, Law M, et al. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care 2007;30:113-9. [Crossref] [PubMed]
- Mugisha JO, Schatz EJ, Randell M, et al. Chronic disease, risk factors and disability in adults aged 50 and above living with and without HIV: findings from the Wellbeing of Older People Study in Uganda. Glob Health Action 2016;9:31098. [Crossref] [PubMed]
- Husain NE, Noor SK, Elmadhoun WM, et al. Diabetes, metabolic syndrome and dyslipidemia in people living with HIV in Africa: re-emerging challenges not to be forgotten. HIV AIDS (Auckl) 2017;9:193-202. [Crossref] [PubMed]
- Njuguna B, Kiplagat J, Bloomfield GS, et al. Prevalence, Risk Factors, and Pathophysiology of Dysglycemia among People Living with HIV in Sub-Saharan Africa. J Diabetes Res 2018;2018:6916497 [Crossref] [PubMed]
- Cohen DB, Allain TJ, Glover S, et al. A survey of the management, control, and complications of diabetes mellitus in patients attending a diabetes clinic in Blantyre, Malawi, an area of high HIV prevalence. Am J Trop Med Hyg 2010;83:575-81. [Crossref] [PubMed]
- Dave JA, Levitt NS, Ross IL, et al. Antiretroviral therapy increases the prevalence of dyslipidaemia in South African HIV-infected patients. PLoS One 2016;11:e0151911 [Crossref] [PubMed]
- Chen D, Misra A. Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab 2002;87:4845-56. [Crossref] [PubMed]
- van der Westhuyzen DR, de Beer FC, Webb NR. HDL cholesterol transport during inflammation. Curr Opin Lipidol 2007;18:147-51. [Crossref] [PubMed]
- van der Voort PH, Gerritsen RT, Bakker AJ, et al. HDL-cholesterol level and cortisol response to synacthen in critically ill patients. Intensive Care Med 2003;29:2199-203. [Crossref] [PubMed]
- Lesi OA, Soyebi KS, Eboh CN. Fatty liver and hyperlipidemia in a cohort of HIV-positive Africans on highly active antiretroviral therapy. J Natl Med Assoc 2009;101:151-5. [Crossref] [PubMed]
- Salami AK, Akande AA, Olokoba AB. Serum lipids and glucose abnor- malities in HIV/AIDS patients on antiretroviral therapies. West Afr J Med 2009;28:10-5. [Crossref] [PubMed]
- Armstrong C, Liu E, Okuma J, et al. Dyslipidemia in an HIV-positive antiretroviral treatment-naive population in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr 2011;57:141-5. [Crossref] [PubMed]
- Bekolo CE, Nguena MB, Ewane L, et al. The lipid profile of HIV-infected patients receiving antiretroviral therapy in a rural Cameroonian population. BMC Public Health 2014;14:236. [Crossref] [PubMed]
- Feleke Y, Fekade D, Mezegebu Y. Prevalence of highly active antiret- roviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop Med J 2012;50:221-30. [PubMed]
- Bittar R, Giral P, Aslangul E, et al. Determinants of low-density lipoprotein particle diameter during antiretroviral therapy including protease inhibitors in HIV-1-infected patients. Antivir Ther 2012;17:855-60. [Crossref] [PubMed]
- Fauvel J, Bonnet E, Ruidavets JB, et al. An interaction between apo C-III variants and protease inhibitors contributes to high triglyceride/low HDL levels in treated HIV patients. AIDS 2001;15:2397-406. [Crossref] [PubMed]
- Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 2010;24:1228-30. [Crossref] [PubMed]
- Lang S, Mary-Krause M, Cotte L, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case- control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med 2010;170:1228-38. [Crossref] [PubMed]
- Sullivan AK, Feher MD, Nelson MR, et al. Marked hypertriglyceridaemia associated with ritonavir therapy. AIDS 1998;12:1393-4. [Crossref] [PubMed]
- Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008;371:1417-26. [Crossref] [PubMed]
- Armstrong C, Liu E, Grinspoon S, et al. Dyslipidemia in an HIV-positive, antiretroviral treatment-naïve population in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr 2011;57:141-5. [Crossref] [PubMed]
- Anyabolu EN. Dyslipidemia in people living with HIV-AIDS in a tertiary hospital in South-East Nigeria. Pan Afr Med J 2017;28:204. [Crossref] [PubMed]
- Floris-Moore M, Howard AA, Lo Y, et al. Increased serum lipids are associated with higher CD4 lymphocyte count in HIV-infected women. HIV Med 2006;7:421-30. [Crossref] [PubMed]
- Menezes de Pádua CA, Moura CS. Availability of data on adverse reactions to antiretroviral drugs in medical charts according to the naranjo algorithm: an example of a Brazilian historical cohort. Clin Drug Investig 2014;34:395-402. [Crossref] [PubMed]
- Misganaw A, Haregu TN, Deribe K, et al. National mortality burden due to communicable, non-communicable, and other diseases in Ethiopia, 1990-2015: findings from the Global Burden of Disease Study 2015. Popul Health Metr 2017;15:29. [Crossref] [PubMed]
- Syed F, Sani, M. Recent advances in HIV-associated cardiovascular diseases in Africa. [online] British Medical Journal. Available online: https://heart-bmj-com.rsm.idm.oclc.org/content/99/16/1146
- Bloomfield GS, Khazanie P, Morris A, et al. HIV and Noncommunicable Cardiovascular and Pulmonary Diseases in Low- and Middle-Income Countries in the ART Era. J Acquir Immune Defic Syndr 2014;67:S40-53. [Crossref] [PubMed]
- Bloomfield G, Hogan J, Keter A, et al. Hypertension and Obesity as Cardiovascular Risk Factors among HIV Seropositive Patients in Western Kenya. PLoS One 2011;6:e22288 [Crossref] [PubMed]
- Niakara A, Drabo Y, Kambire Y, et al. Cardiovascular diseases and HIV infection: study of 79 cases at the National Hospital of Ouagadougou (Burkina Faso). Bull Soc Pathol Exot 2002;95:23-6. [PubMed]
- Ntsekhe M, Hakim J. Impact of Human Immunodeficiency Virus Infection on Cardiovascular Disease in Africa. Circulation 2005;112:3602-7. [Crossref] [PubMed]
- Murphy A, Biringanine M, Roberts B, et al. Diabetes care in a complex humanitarian emergency setting: a qualitative evaluation. BMC Health Serv Res 2017;17:431. [Crossref] [PubMed]
- Ahmed MH, Husain NE, Almobarak AO. Nonalcoholic Fatty Liver Disease and Risk of Diabetes and Cardiovascular Disease: What Is Important for Primary Care Physicians? J Family Med Prim Care 2015;4:45-52. [Crossref] [PubMed]
- Bani-Sadr F, Barange K, Daoud F, et al. Persistently normal alanine aminotransferase levels in HIV/HCV- coinfected patients: the role of steatosis. HIV Med 2009;10:417-21. [Crossref] [PubMed]
- Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in patients co infected with human immunodeficiency virus/hepatitis C virus: a meta- analysis of the risk factors. Hepatology 2010;52:71-8. [Crossref] [PubMed]
- Sterling RK, Smith PG, Brunt EM. Hepatic Steatosis in HIV: A Prospective Study in Patients without Viral Hepatitis, Diabetes, or Alcohol Abuse. J Clin Gastroenterol 2013;47:182-7. [Crossref] [PubMed]
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274-85. [Crossref] [PubMed]
- Han TM, Naicker S, Ramdial PK, et al. A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int 2006;69:2243-50. [Crossref] [PubMed]
- Wyatt CM, Klotman P. HIV-Associated Nephropathy in the Era of Antiretroviral Therapy. Am J Med 2007;120:488-92. [Crossref] [PubMed]
- Husain NE, Ahmed M, Almobarak A, et al. HIV-Associated Nephorpathy in Africa: Pathology, Clinical Presentation and Strategy for Prevention. J Clin Med Res 2018;10:1-8. [PubMed]
- Fabian J, Naicker S. HIV and kidney disease in sub-Saharan Africa. Nat Rev Nephrol 2009;5:591-8. [Crossref] [PubMed]
- Gerntholtz TE, Goetsch S, Katz I. HIV-related nephropathy: A South African perspective. Kidney Int 2006;69:1885-91. [Crossref] [PubMed]
- Arendse CG, Wearne N, Okpechi I, et al. The acute, the chronic and the news of HIV-related renal disease in Africa. Kidney Int 2010;78:239-45. [Crossref] [PubMed]
- Ibrahim F, Hamzah L, Jones R, et al. Baseline Kidney Function as Predictor of Mortality and Kidney Disease Progression in HIV-Positive Patients. Am J Kidney Dis 2012;60:539-47. [Crossref] [PubMed]
- Mulenga LB, Kruse G, Lakhi S, et al. Baseline renal insufficiency and risk of death among HIV-infected adults on antiretroviral therapy in Lusaka, Zambia. AIDS 2008;22:1821-7. [Crossref] [PubMed]
- Sinha U, Mukhopadhyay P, Sengupta N, et al. Human immunodeficiency virus endocrinopathy. Indian J Endocrinol Metab 2011;15:251. [Crossref] [PubMed]
- Ekpebegh CO, Ogbera AO, Longo-Mbenza B, et al. Basal Cortisol Levels and Correlates of Hypoadrenalism in Patients with Human Immunodeficiency Virus Infection. Med Princ Pract 2011;20:525-9. [Crossref] [PubMed]
- Odeniyi IA, Fasanmade O, Ajala M, et al. Adrenocortical Function in Nigerians With Human Immunodeficiency Virus. Ghana Med J 2013;47:171-7. [PubMed]
- Cournil A, Eymard-Duvernay S, Diouf A, et al. Reduced Quantitative Ultrasound Bone Mineral Density in HIV-Infected Patients on Antiretroviral Therapy in Senegal. PLoS One 2012;7. [PubMed]
- Ukibe NR, Ukibe S, Emelumadu O, et al. Impact of thyroid function abnormalities on reproductive hormones during menstrual cycle in premenopausal HIV infected females at NAUTH, Nnewi, Nigeria. PLoS One 2017;12:e0176361 [Crossref] [PubMed]
- Meya DB, Katabira E, Otim M, et al. Functional adrenal insufficiency among critically ill patients with human immunodeficiency virus in a resource-limited setting. Afr Health Sci 2007;7:101-7. [PubMed]
- Long LC, Fox M, Sauls C, et al. The High Cost of HIV-Positive Inpatient Care at an Urban Hospital in Johannesburg, South Africa. PLoS One 2016;11:e0148546 [Crossref] [PubMed]
Cite this article as: Ahmed MH, Gooneratne S, Bondje S, Rawther F, Duku A, Mital D. HIV metabolic clinic: can the journey start from a UK clinic to a sub-Saharan African nation? J Public Health Emerg 2018;2:28.


